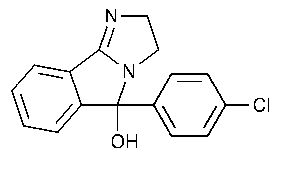
Mazindol
3H-Imidazo[2,1-a]isoindol-5-ol,5-(4-chlorophenyl)-2,5-dihydro-,(±)-.
(±)-5-(p-Chlorophenyl)-2,5-dihydro-3H-imidazo[2,1-a]isoindol-5-ol [22232-71-9].
»Mazindol contains not less than 98.0percent and not more than 102.0percent of C16H13ClN2O,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Clarity and color of solution—
A1in 100solution of Mazindol in a mixture of chloroform and methanol (9:1)is clear and not darker in color than a solution prepared by mixing equal volumes of Matching Fluid C(see Color and Achromicity á631ñ)and water.
Identification—
A:
Infrared Absorption á197Kñ.
B:
Ultraviolet Absorption á197Uñ—
Solution:
10µg per mL.
Medium:
0.6Nhydrochloric acid.
Absorptivities at 272nm,calculated on the dried basis,do not differ by more than 3.0%.
Loss on drying á731ñ—
Dry it in vacuum at 60 for 2hours:it loses not more than 0.5%of its weight.
for 2hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.002%.
Sulfate á221ñ—
Triturate a 500-mg portion with 10mLof water in a mortar.Filter the suspension through a water-washed filter,and rinse the mortar and filter with 30mLof water,collecting the combined filtrate and washings in a 50-mLcolor-comparison tube.The filtrate shows no more sulfate than corresponds to 0.20mLof 0.020Nsulfuric acid (0.04%).
Chromatographic purity—
Dissolve 10mg in 2.0mLof a mixture of chloroform and methanol (9:1)to obtain the test solution.Dissolve a suitable quantity of USP Mazindol RSin a mixture of chloroform and methanol (9:1)to obtain a Standard solution having a concentration of 5.0mg per mL.Dilute portions of this solution quantitatively and stepwise with the mixture of chloroform and methanol (9:1)to obtain a series of diluted standard solutions having concentrations of 0.100,0.050,0.025,and 0.0125mg per mL,respectively.Separately apply a 20-µLportion of the test solution and 20-µLportions of the Standard solution and each diluted standard solution to a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Allow the spots to dry,and develop the chromatogram in a solvent system consisting of a mixture of chloroform,alcohol,and ammonium hydroxide (80:20:1)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,and allow the solvent to evaporate.Locate the spots on the plate by examination under short-wavelength UVlight:the chromatograms show principal spots at about the same RFvalue.Estimate the concentration of any secondary spots present in the chromatogram from the test solution by comparison with the diluted standard solutions:the principal spots from the 0.100,0.050,0.025,and 0.0125mg per mLdilutions are equivalent to 2.0%,1.0%,0.50%,and 0.25%of impurities,respectively.No individual impurity is greater than 1.0%,and the sum of the impurities is not greater than 2.0%.
Assay—
Transfer about 230mg of Mazindol,accurately weighed,to a suitable flask,dissolve in 40mLof glacial acetic acid,add 3drops of crystal violet TS,and titrate with 0.1Nperchloric acid VSto an emerald-green endpoint.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 28.47mg of C16H13ClN2O.
Auxiliary Information—
Staff Liaison:Daniel K.Bempong,Ph.D.,Scientist
Expert Committee:(PA2)Pharmaceutical Analysis 2
USP28–NF23Page 1186
Phone Number:1-301-816-8143