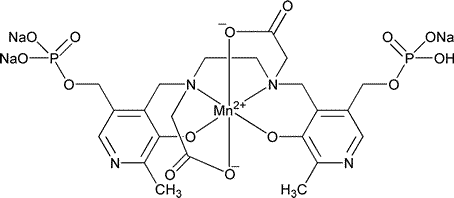
Mangafodipir Trisodium
C22H27MnN4Na3O14P2
757.33
Trisodium trihydrogen (OC-6-13)-[[N,N¢-1,2-ethanediylbis[N-[[3-hydroxy-2-methyl-5-[(phosphonooxy)methyl]-4-pyridinyl]methyl]glycinato]](8-)]manganate(6-).
Trisodium trihydrogen (OC-6-13)-[[N,N¢-ethylenebis[N-[[3-hydroxy-5-(hydroxymethyl)-2-methyl-4-pyridyl]methyl]glycine]5,5¢-bis(phosphato)](8-)]manganate(6-) [140678-14-4].
Trisodium trihydrogen (OC-6-13)-[[N,N¢-1,2-ethanediylbis[N-[[3-hydroxy-2-methyl-5-[(phosphonooxy)methyl]-4-pyridinyl]methyl]glycinato]](8-)]manganate(6-).
Trisodium trihydrogen (OC-6-13)-[[N,N¢-ethylenebis[N-[[3-hydroxy-5-(hydroxymethyl)-2-methyl-4-pyridyl]methyl]glycine]5,5¢-bis(phosphato)](8-)]manganate(6-) [140678-14-4].
»Mangafodipir Trisodium contains not less than 97.0percent and not more than 103.0percent of C22H27MnN4Na3O14P2,calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed containers.Store at 25 ,excursions permitted between 15
,excursions permitted between 15 and 30
and 30 .
.
USP Reference standards á11ñ—
USP Endotoxin RS.USP Mangafodipir Related Compound A RS.USP Mangafodipir Related Compound B RS.USP Mangafodipir Related Compound C RS.USP Mangafodipir Trisodium RS.
Identification—
Microbial limits á61ñ—
The total aerobic microbial count is not more than 500cfu per g.
Bacterial endotoxins á85ñ:
not more than 0.13USP Endotoxin Unit per mg.
pHá791ñ:
between 5.5and 7.0,in a solution (1in 100).
Water,Method Iá921ñ:
not more than 20%.
Limit of residual solvents—
Internal standard solution—
Transfer 600µLof methyl ethyl ketone to a 100-mLvolumetric flask,dilute with water to volume,and mix to obtain a solution having a concentration of about 5mg per mL.Transfer 2mLof this solution to a 100-mLvolumetric flask,dilute with water to volume,and mix to obtain a solution having a known concentration of about 0.1mg per mL.
Standard stock solution—
Transfer about 1g of dehydrated alcohol and 1g of acetone,both accurately weighed,to a 100-mLvolumetric flask,and dilute with water to volume.Transfer 10.0mLof this solution to a 100-mLvolumetric flask,dilute with water to volume,and mix to obtain a solution having a known concentration of about 1mg each of alcohol and acetone per mL.
Standard solutions—
Transfer 10.0mLofInternal standard solution to each of four 100-mLvolumetric flasks.Separately add 0mL,1.0mL,5.0mL,and 10.0mLofStandard stock solution to the volumetric flasks,and dilute each with water to volume to obtain solutions having known concentrations of 0.0µg per mLand about 10µg per mL,50µg per mL,and 100µg per mLeach of alcohol and acetone,respectively.Add 7.0mLof eachStandard solution to separate headspace sample vials,and cap.
Test solution—
Transfer about 1g of Mangafodipir Trisodium,accurately weighed,to a sample vial,add 7.0mLof theStandard solution having a concentration of 0.0µg per mL,cap,and swirl to dissolve.
Chromatographic system(see Chromatography á621ñ)—
The gas chromatograph is equipped with a flame-ionization detector,a 0.32-mm ×30-m fused silica column coated with 1.8-µm G43stationary phase.The carrier gas is helium,flowing at a rate of 1.5mLper minute.The temperatures of the injection port and the oven are maintained at 150 and 50
and 50 ,respectively.The bath temperature for the headspace sample vials is maintained at 90
,respectively.The bath temperature for the headspace sample vials is maintained at 90 ,the valve/loop temperature is maintained at 130
,the valve/loop temperature is maintained at 130 ,and the sample thermostating time is 15minutes.Chromatograph theStandard solutions,and record the peak responses as directed forProcedure:the resolution,R,between alcohol and acetone is not less than 5;and the relative standard deviation for replicate injections,determined from the peak response ratios of the analyte to the internal standard,is not more than 2.0%.Calculate the peak response ratios of the analyte to the internal standard,and plot the results.Determine the linear regression equation of the standards by the mean-square method,and record the linear regression equation and the correlation coefficient.Asuitable system is one that yields a line having a correlation coefficient of not less than 0.990.
,and the sample thermostating time is 15minutes.Chromatograph theStandard solutions,and record the peak responses as directed forProcedure:the resolution,R,between alcohol and acetone is not less than 5;and the relative standard deviation for replicate injections,determined from the peak response ratios of the analyte to the internal standard,is not more than 2.0%.Calculate the peak response ratios of the analyte to the internal standard,and plot the results.Determine the linear regression equation of the standards by the mean-square method,and record the linear regression equation and the correlation coefficient.Asuitable system is one that yields a line having a correlation coefficient of not less than 0.990.
Procedure—
Separately inject equal volumes (about 1mL)of the gaseous headspace of each of theStandard solutions and theTest solution into the chromatograph,record the chromatograms,and measure the peak responses.Calculate the percentages (w/w)of alcohol and acetone in the portion of Mangafodipir Trisodium taken by the formula:
(7/10,000)(C/W),
in whichCis the concentration,in µg per mL,of alcohol or acetone in theTest solution,as determined from the relevant standard response line;andWis the weight,in g,of Mangafodipir Trisodium taken:not more than 0.1%of alcohol is found;and not more than 0.01%of acetone is found,both calculated on the anhydrous basis.
Limit of free manganese and free fodipir—
Ascorbic acid solution—
Dissolve 0.5g of ascorbic acid in 10mLof water.
Manganese solution—
Transfer about 3.6g of manganese chloride,accurately weighed,to a 1000-mLvolumetric flask,dissolve in and dilute with 0.1Nhydrochloric acid to volume,and mix.Transfer 100.0mLof this solution to a 500-mLvolumetric flask,dilute with water to volume,and mix.
Edetate titrant solution—
Transfer about 37g of edetate disodium,accurately weighed,to a 1000-mLvolumetric flask,dilute with water to volume,and mix.Transfer 36mLof this solution to a 1000-mLvolumetric flask,dilute with water to volume,and mix to obtain a solution having a concentration of 0.0036moles per L.
STANDARDIZATION OF0.0036M EDETATE TITRANT SOLUTION—
Accurately weigh about 200mg of chelometric standard calcium carbonate,previously dried at 110 for 2hours and cooled in a desiccator,transfer to a 100-mLvolumetric flask,and add 10mLof water and about 4mLof diluted hydrochloric acid.Swirl the flask to dissolve,dilute with water to volume,and mix.Transfer 5.0mLof this solution to a beaker while stirring,preferably with a magnetic stirrer;and add about 15mLof sodium hydroxide TSand enough hydroxynaphthol blue indicator to achieve a percent transmission of about 95%,using a suitable autotitrator at a wavelength of 620nm,calibrated to 100%transmission with water.Add 20.0mLofEdetate titrant solution,and continue to titrate until 3mLof titrant have been added beyond the sharp break point,as determined from the titration curve obtained by plotting relative transmittance versus volume,in mL,of titrant added.Determine the endpoint volume from the titration curve.The final titration volume is the sum of the endpoint volume and the 20.0mLofEdetate titrant solution initially added.Calculate the molarity of theEdetate titrant solution by the formula:
for 2hours and cooled in a desiccator,transfer to a 100-mLvolumetric flask,and add 10mLof water and about 4mLof diluted hydrochloric acid.Swirl the flask to dissolve,dilute with water to volume,and mix.Transfer 5.0mLof this solution to a beaker while stirring,preferably with a magnetic stirrer;and add about 15mLof sodium hydroxide TSand enough hydroxynaphthol blue indicator to achieve a percent transmission of about 95%,using a suitable autotitrator at a wavelength of 620nm,calibrated to 100%transmission with water.Add 20.0mLofEdetate titrant solution,and continue to titrate until 3mLof titrant have been added beyond the sharp break point,as determined from the titration curve obtained by plotting relative transmittance versus volume,in mL,of titrant added.Determine the endpoint volume from the titration curve.The final titration volume is the sum of the endpoint volume and the 20.0mLofEdetate titrant solution initially added.Calculate the molarity of theEdetate titrant solution by the formula:
(5/100.09)(W)/(100V),
in which 100.09is the molecular weight of calcium carbonate;Wis the weight,in mg,of the calcium carbonate taken;andVis the final titration volume,in mL,ofEdetate titrant solution.
Procedure—
Transfer about 1g of Mangafodipir Trisodium,accurately weighed,to a suitable beaker,add about 100mLof water,1.0mLofAscorbic acid solution,10mLof ammonia–ammonium chloride buffer TS,0.1mLof eriochrome black TS,and 1.0mLofManganese solution,and record the color.If the color is yellow to green,add additional 1.0-mLincrements ofManganese solution until the color is red.Record the volume added.Titrate with theEdetate titrant solution,determining the endpoint photometrically.Perform a blank determination,and make any necessary correction (seeTitrimetry á541ñ).Calculate the percentage of free manganese in the portion of Mangafodipir Trisodium taken by the formula:
5.49V(M/W),
in whichVis the volume,in mL,of theEdetate titrant solution;Mis the molarity of theEdetate titrant solution;andWis the weight,in g,of Mangafodipir Trisodium taken.Calculate the percentage of free fodipir in the portion of Mangafodipir Trisodium taken by the formula:
63.85V(M/W),
in whichV,M,andWare as defined herein:not more than 0.03%of free manganese is found;and not more than 0.5%of free fodipir is found,both calculated on the anhydrous basis.
Related compounds—
Ascorbic acid solution—
Dissolve 0.4g of ascorbic acid in 100mLof water.
Phosphate buffer—
Prepare as directed in theAssay.
Mobile phase—
Prepare as directed in theAssay.[NOTE—Increasing the proportion of acetonitrile will decrease the retention times.]
System suitability stock solution—
Prepare as directed forStandard stock preparation in theAssay.
System suitability solution 1—
Prepare a solution of USP Mangafodipir Trisodium RShaving a known concentration of about 4.0mg per mL.Transfer 5.0mLof this solution to a 50-mLvolumetric flask,add 5.0mLofSystem suitability stock solution,5.0mLofPhosphate buffer,and 5.0mLofAscorbic acid solution.Dilute with nitrogen-purged water to volume,and mix to obtain a solution having a concentration of about 0.4mg of USP Mangafodipir Trisodium RS,and about 0.01mg each of USP Mangafodipir Related Compound A RSand USP Mangafodipir Related Compound B RSper mL.[NOTE—Store in a refrigerator and under nitrogen to avoid excessive exposure to heat,air,and light.]
System suitability solution 2—
Transfer about 10mg of USP Mangafodipir Related Compound C RSto a 100-mLvolumetric flask,dilute with water to volume,and mix.Transfer 5.0mLof this solution to a 50-mLvolumetric flask,and add 5.0mLofPhosphate buffer.
Test solution—
Transfer an accurately weighed quantity of Mangafodipir Trisodium,equivalent to about 100mg of mangafodipir trisodium,to a 50-mLvolumetric flask,dilute with water to volume,and mix.Transfer 10.0mLof this solution to a second 50-mLvolumetric flask,add 5.0mLofPhosphate buffer,dilute with water to volume,and mix.[NOTE—Store in a refrigerator and under nitrogen to avoid excessive exposure to heat,air,and light.]
Chromatographic system(see Chromatography á621ñ)—
Prepare as directed in theAssay.ChromatographSystem suitability solution 2,and record the peak responses as directed forProcedure:note the elution time to identify the mangafodipir related compound Cpeak,if present,in the chromatogram ofSystem suitability solution 1.ChromatographSystem suitability solution 1,and record the peak responses as directed forProcedure:the retention time for mangafodipir is between 18and 30minutes.The peak area for mangafodipir related compound Cis less than 0.1%.[NOTE—If the peak area is more than 0.1%of the total of all peak areas,prepare fresh quantities ofAscorbic acid solution andSystem suitability solution 1,and repeat the test.If the peak area of mangafodipir related compound Cis still greater than 0.1%,repeat the test using another column.Acontaminated column can result in oxidation of Mn(II)to Mn(III),forming related compound C.]The tailing factor for the mangafodipir peak is not more than 2.3;the column efficiency is not less than 1000theoretical plates;the resolution,R,between mangafodipir related compound Band mangafodipir is not less than 1.5;and the relative standard deviation for replicate injections is not more than 10%for each peak.[NOTE—If the resolution is less than 1.5,adjust the Mobile phaseby increasing the concentration of tetrabutylammonium hydrogen sulfate.]
Procedure—
Inject about 10µLof theTest solution into the chromatograph,record the chromatogram,and measure the areas for all the major peaks.The relative retention times for ascorbic acid,mangafodipir related compound A,Mn(II)-5-methyl dipyridoxal monophosphate (Mn(II)-5-methyl DPMP)if present,mangafodipir related compound C,mangafodipir related compound B,and mangafodipir are about 0.1,0.3,0.4,0.6,0.8,and 1.0,respectively.Calculate the percentages of mangafodipir related compound A,mangafodipir related compound B,mangafodipir related compound C,and Mn(II)-5-methyl DPMPin the portion of Mangafodipir Trisodium taken by the formula:
100(ri/rs),
in which riis the peak area of each impurity;andrsis the sum of the areas of all of the peaks:not more than 0.5%each of mangafodipir related compound Aand mangafodipir related compound Bis found;not more than 0.6%of mangafodipir related compound Cis found;not more than 0.3%of Mn(II)-5-methyl DPMPis found;not more than 0.3%of any other impurity is found;not more than a total of 0.5%of other impurities is found;and not more than a total of 2.0%of impurities is found.
Assay—
Phosphate buffer—
Transfer about 26.8g of dibasic sodium phosphate to a 1000-mLvolumetric flask,add 900mLof water,and adjust with 1Nsodium hydroxide or 1Nhydrochloric acid to a pHof about 8.0.Dilute with water to volume,filter,and degas.
Mobile phase—
Transfer about 0.61g of boric acid and 9.2g of tetrabutylammonium hydrogen sulfate to a 1000-mLvolumetric flask,add 640mLwater,and mix.Adjust with 3Nsodium hydroxide to a pHof about 9.3,add 250mLof acetonitrile,dilute with water to volume,and mix.Adjust with 3Nhydrochloric acid or 3Nsodium hydroxide to a pHof about 10.5,filter,and degas.Make adjustments if necessary (seeSystem Suitability underChromatography á621ñ).
Standard stock preparation—
Transfer about 10mg each of USP Mangafodipir Related Compound A RSand USP Mangafodipir Related Compound B RS,both accurately weighed,to a 100-mLvolumetric flask,dilute with water to volume,and mix.
Standard preparation—
Transfer about 100mg of USP Mangafodipir Trisodium RSto a 50-mLvolumetric flask,dilute with water to volume,and mix.Transfer 10.0mLof this solution to a 50-mLvolumetric flask,add 5.0mLofStandard stock preparation and 5.0mLofPhosphate buffer,dilute with water to volume,and mix.[NOTE—Store in a refrigerator and under nitrogen to avoid exposure to excessive heat,air,or light.]
Assay preparation—
Transfer an accurately measured quantity of Mangafodipir Trisodium,equivalent to about 100mg of mangafodipir trisodium,to a 50-mLvolumetric flask,dilute with water to volume,and mix.Transfer 10.0mLof this solution to a 50-mLvolumetric flask,add 5.0mLofPhosphate buffer,dilute with water to volume,and mix.[NOTE—Store in a refrigerator and under nitrogen to avoid exposure to excessive heat,air,or light.]
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 310-nm detector and a 4.6-mm ×15-cm column that contains 5-µm packing L21.The chromatograph is maintained at about 20 .The flow rate is 0.8mLper minute.Chromatograph theStandard preparation,and record the peak responses as directed forProcedure:the resolution,R,between mangafodipir related compound Aand mangafodipir related compound Bis not less than 1.5;the column efficiency is not less than 1000theoretical plates;and the tailing factor is not more than 2.3.
.The flow rate is 0.8mLper minute.Chromatograph theStandard preparation,and record the peak responses as directed forProcedure:the resolution,R,between mangafodipir related compound Aand mangafodipir related compound Bis not less than 1.5;the column efficiency is not less than 1000theoretical plates;and the tailing factor is not more than 2.3.
Procedure—
Separately inject equal volumes (about 10µL)of theStandard preparation and theAssay preparation into the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the percentage of C22H27MnN4Na3O14P2in the portion of Mangafodipir Trisodium taken by the formula:
25,000(C/W)(rU/rS),
in whichCis the concentration,in mg per mL,of USP Mangafodipir Trisodium RSin theStandard preparation;Wis the weight,in mg,of the Mangafodipir Trisodium taken;andrUandrSare the peak responses obtained from theAssay preparation and theStandard preparation,respectively.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(RMI)Radiopharmaceuticals and Medical Imaging Agents
USP28–NF23Page 1178
Pharmacopeial Forum:Volume No.29(6)Page 1921
Phone Number:1-301-816-8305