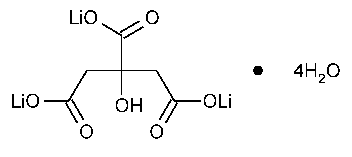
Lithium Citrate
1,2,3-Propanetricarboxylic acid,2-hydroxy-trilithium salt tetrahydrate.
Trilithium citrate tetrahydrate [6080-58-6].
Anhydrous 209.93 [919-16-4].
»Lithium Citrate contains not less than 98.0percent and not more than 102.0percent of C6H5Li3O7,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
When moistened with hydrochloric acid,it imparts an intense crimson color to a nonluminous flame.
B:
It responds to the test for Citrate á191ñ.
pHá791ñ:
between 7.0and 10.0,in a solution (1in 20).
Water,Method IIIá921ñ—
Dry it at 150 for 3hours:it loses between 24.0%and 28.0%of its weight.
for 3hours:it loses between 24.0%and 28.0%of its weight.
Carbonate—
Add about 0.5g to 5mLof 6Nacetic acid:not more than a slight effervescence is produced.
Heavy metals á231ñ—
Dissolve 2.0g in 2mLof 0.1Nhydrochloric acid,and dilute with water to 25mL:the limit is 0.001%.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent:
Dimethyl sulfoxide.
Assay—
Standard preparation—
Transfer to a 100-mLvolumetric flask about 30mg of USP Lithium Carbonate RS,accurately weighed.Add about 20mLof water and 0.5mLof hydrochloric acid,shake until dissolved,dilute with water to volume,and mix.Pipet 20mLof the resulting solution into a 1000-mLvolumetric flask,add about 800mLof water and 20mLof a suitable surfactant solution,appropriately diluted,dilute with water to volume,and mix.
Assay preparation—
Transfer about 800mg of Lithium Citrate,accurately weighed,to a 1000-mLvolumetric flask.Dissolve in water,add 0.5mLof hydrochloric acid,dilute with water to volume,and mix.Pipet 20mLof this solution into a 1000-mLvolumetric flask,add about 800mLof water and 20mLof the surfactant solution,dilute with water to volume,and mix.
Procedure—
Employ a suitable flame photometer,and adjust the instrument with the surfactant solution.Aspirate into the instrument the Standard preparationand the Assay preparation,and measure the emission at about 671nm.Calculate the quantity,in mg,of C6H5Li3O7in the portion of Lithium Citrate taken by the formula:
(419.84/221.67)(50C)(A/S),
in which 419.84is twice the molecular weight of anhydrous lithium citrate,221.67is three times the molecular weight of lithium carbonate,Cis the concentration,in µg per mL,of USP Lithium Carbonate RSin the Standard preparation,and Aand Sare the photometer readings of the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Ravi Ravichandran,Ph.D.,Senior Scientist
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 1143
Phone Number:1-301-816-8330