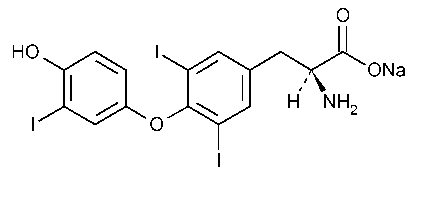
Liothyronine Sodium
L-Tyrosine,O-(4-hydroxy-3-iodophenyl)-3,5-diiodo-,monosodium salt.
Monosodium L-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]alanine [55-06-1].
»Liothyronine Sodium is the sodium salt of L-3,3¢,5-triiodothyronine.It contains not less than 95.0percent and not more than 101.0percent of C15H11I3NNaO4,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
The UVabsorption spectrum of a 1in 10,000solution in dilute hydrochloric acid (1in 50)in 80percent alcohol exhibits maxima at the same wavelengths as that of a similar solution of USP Liothyronine RS,concomitantly measured,and the respective absorptivities,both calculated on the dried basis in terms of the acid,at the wavelength of maximum absorbance at about 297nm do not differ by more than 5.0%.
B:
Heat about 50mg with a few drops of sulfuric acid in a porcelain crucible:violet vapors of iodine are evolved.
C:
The residue from the ignition of it responds to the tests for Sodium á191ñ.
Specific rotation á781Sñ:
between +18 and +22
and +22 .
.
Test solution:
20mg per mL,in a mixture of alcohol and 1.2Nhydrochloric acid (4:1).
Loss on drying á731ñ—
Dry it at 105 for 2hours:it loses not more than 4.0%of its weight.
for 2hours:it loses not more than 4.0%of its weight.
Limit of inorganic iodide—
Extracting solution,Reference solution,and Electrode system—
Proceed as directed in the Limit of inorganic iodidetest under Levothyroxine Sodium.
Test solution—
Transfer 7.5mg of Liothyronine Sodium to a beaker,add 100mLof Extracting solution,and sonicate for 5minutes.
Procedure—
Proceed as directed in the Limit of inorganic iodidetest under Levothyroxine Sodium:the limit is 0.08%.
Limit of levothyroxine sodium—
Mobile phaseand Chromatographic system—
Proceed as directed in the Assay.
Standard solution—
Proceed as directed for Standard preparationin the Assay.
Test solution—
Proceed as directed for Assay preparationin the Assay.
Procedure—
Separately inject equal volumes (about 100µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the levothyroxine peak responses.Calculate the quantity,in µg,of levothyroxine sodium (C15H10I4NNaO4)in the portion of Liothyronine Sodium taken by the formula:
(798.85/776.87)(10C)(rU/rS),
in which 798.85and 776.87are the molecular weights of levothyroxine sodium and levothyroxine,respectively;Cis the concentration,in µg per mL,of USP Levothyroxine RSin the Standard solution;andrUand rSare the levothyroxine peak responses obtained from the Test solutionand the Standard solution,respectively:not more than 5.0%of levothyroxine sodium is found.
Chloride content—
Weigh accurately 100mg,previously dried,and transfer to a platinum dish.Ignite over a low flame,protecting the dish from air currents during the ignition.When carbonization is complete,cool the dish,add 2drops of water,and break up the charred mass thoroughly with a stirring rod.Add 10mLof water and 5mLof ammonium hydroxide,and mix.Transfer the slurry to a glass-stoppered,50-mLflask,and wash the platinum dish and the stirring rod with water,adding the washings to the flask,until the volume of the solution is about 25mL.Add 10mLof silver nitrate solution (1in 20),shake thoroughly,and filter through a retentive paper into a 50-mLcolor-comparison tube.Wash the flask and the filter paper with 10mLof water,and add the washings to the tube.Acidify the combined filtrate and washings to litmus with nitric acid,and dilute with water to 50mL.Prepare a control by mixing 5mLof ammonium hydroxide,20mLof water,and 10mLof silver nitrate solution (1in 20),filtering the mixture through a retentive paper into a 50-mLcolor-comparison tube,then washing the filter paper with 10mLof water into the tube,acidifying the contents of the tube to litmus with nitric acid,diluting with water to 50mL,and adding sodium chloride solution (1in 1000)in 0.1-mLincrements until the turbidity of the control matches that of the test solution.Not more than 2.0mLof sodium chloride is required (1.2%).
Sodium content—
Weigh accurately about 100mg,previously dried,and transfer to a platinum dish.Add 8to 10drops of sulfuric acid,and ignite to constant weight,taking care to avoid spattering.Each mg of residue is equivalent to 0.324mg of Na.Correct the result for the amount of sodium equivalent to the NaCl found in the test for Chloride content:not less than 2.9%and not more than 4.0%is found.
Assay—
Mobile phase—
Prepare a filtered and degassed mixture of water and acetonitrile (60:40)that contains 0.5mLof phosphoric acid in each 1000mL.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Transfer accurately weighed quantities of USP Liothyronine RSand USP Levothyroxine RS,and dissolve in and dilute quantitatively and stepwise with Mobile phaseto obtain a solution having known concentrations of about 10µg of liothyronine per mLand 0.5µg of levothyroxine per mL.
Assay preparation—
Transfer an accurately weighed portion of 100µg of Liothyronine Sodium to a centrifuge tube,add 2glass beads,pipet 10mLof Mobile phaseinto the tube,and mix using a vortex mixer for 3minutes.Centrifuge to obtain a clear supernatant,filtering if necessary.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 225-nm detector and a 4.6-mm ×25-cm column that contains packing L10.The flow rate is about 1.5mLper minute.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the resolution,R,between the levothyroxine and liothyronine peaks is not less than 5.0;and the relative standard deviation for replicate injections is not more than 2.0%for liothyronine.
Procedure—
Separately inject equal volumes (about 100µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in µg,of C15H11I3NNaO4in the portion of Liothyronine Sodium taken by the formula:
(672.96/650.97)(10C)(rU/rS),
in which 672.96and 650.97are the molecular weights of liothyronine sodium and liothyronine,respectively;Cis the concentration,in µg per mL,of USP Liothyronine RSin the Standard preparation;and rUand rSare the liothyronine peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Elena Gonikberg,Ph.D.,Scientist
Expert Committee:(PA4)Pharmaceutical Analysis 4
USP28–NF23Page 1137
Pharmacopeial Forum:Volume No.30(5)Page 1631
Phone Number:1-301-816-8251