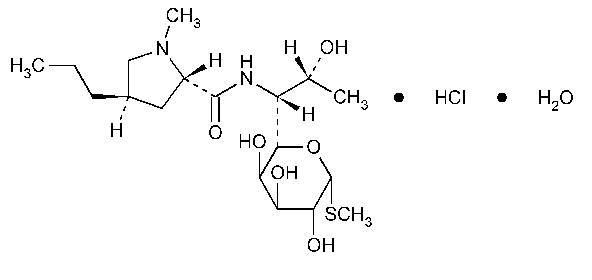
Lincomycin Hydrochloride
D-erythro-a-D-galacto-Octopyranoside,methyl 6,8-dideoxy-6-[[(1-methyl-4-propyl-2-pyrrolidinyl)carbonyl]amino]-1-thio-,monohydrochloride,monohydrate,(2S-trans)-.
Methyl 6,8-dideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-D-erythro-a-D-galacto-octopyranoside monohydrochloride monohydrate [7179-49-9].
Anhydrous 443.01 [859-18-7].
»Lincomycin Hydrochloride has a potency equivalent to not less than 790µg of lincomycin (C18H34N2O6S)per mg.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification,Infrared Absorption á197Mñ.
Specific rotation á781Sñ:
between +135 and +150
and +150 .
.
Test solution:
20mg per mL,in water.
Crystallinity á695ñ:
meets the requirements.
pHá791ñ:
between 3.0and 5.5,in a solution (1in 10).
Water,Method Iá921ñ:
between 3.0%and 6.0%.
Limit of lincomycin B—
Use the chromatogram obtained from the Assay preparationin the Assay:the area of the lincomycin Bpeak is not greater than 5.0%of the sum of the areas of the lincomycin Bpeak and the lincomycin peak.
Other requirements—
Where the label states that Lincomycin Hydrochloride is sterile,it meets the requirements for Sterilityand Bacterial endotoxinsunder Lincomycin Injection.Where the label states that Lincomycin Hydrochloride must be subjected to further processing during the preparation of injectable dosage forms,it meets the requirements for Bacterial endotoxinsunder Lincomycin Injection.
Assay—
Mobile phase—
Add 13.5mLof phosphoric acid to 1000mLof water,and adjust with ammonium hydroxide to a pHof 6.0.Prepare a filtered and degassed mixture of this solution,acetonitrile,and methanol (780:150:150).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Dissolve an accurately weighed quantity of USP Lincomycin Hydrochloride RSin Mobile phaseto obtain a solution having a known concentration of about 1.2mg per mL,using sonication if necessary to effect solution.
Assay preparation—
To about 12mg of Lincomycin Hydrochloride,accurately weighed,add 10.0mLof Mobile phase.Shake by mechanical means for 5minutes,and sonicate if necessary to effect solution.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 210-nm detector and a 4.6-mm ×25-cm column that contains 5-µm packing L7and is maintained at a temperature of 46 .The flow rate is about 1mLper minute.Chromatograph the Standard preparation,and record the responses as directed for Procedure:the tailing factor for the main lincomycin peak is not more than 1.3;the column efficiency determined from the main lincomycin peak is not less than 4000theoretical plates;and the relative standard deviation for replicate injections is not more than 2.0%.
.The flow rate is about 1mLper minute.Chromatograph the Standard preparation,and record the responses as directed for Procedure:the tailing factor for the main lincomycin peak is not more than 1.3;the column efficiency determined from the main lincomycin peak is not less than 4000theoretical plates;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the areas for the major peaks.The relative retention times are about 0.5for lincomycin Band 1.0for lincomycin.Calculate the quantity,in µg,of lincomycin (C18H34N2O6S)in each mg of the Lincomycin Hydrochloride taken by the formula:
10(CP/W)(rU/rS),
in which Cis the concentration,in mg per mL,of USP Lincomycin Hydrochloride RSin the Standard preparation;Pis the designated potency,in µg of lincomycin per mg,of USP Lincomycin Hydrochloride RS;Wis the weight,in mg,of the portion of Lincomycin Hydrochloride taken to prepare the Assay preparation;and rUand rSare the lincomycin peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1133
Phone Number:1-301-816-8335