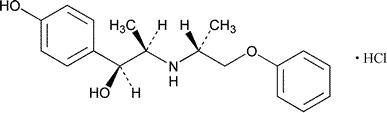
Isoxsuprine Hydrochloride
Benzenemethanol,4-hydroxy-a-[1-[(1-methyl-2-phenoxyethyl)amino]ethyl]-,hydrochloride,stereoisomer.
p-Hydroxy-a-[1-[(1-methyl-2-phenoxyethyl)amino]ethyl]benzyl alcohol hydrochloride.
(±)-(aR*)-p-Hydroxy-a-[(1S*)-1-[[(1S*)-1-methyl-2-phenoxyethyl]amino]ethyl]benzyl alcohol hydrochloride [579-56-6;34331-89-0].
»Isoxsuprine Hydrochloride contains not less than 97.0percent and not more than 103.0percent of C18H23NO3·HCl,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
Infrared Absorption á197Kñ.
C:
To 1mLof a solution (1in 100),obtained by heating as necessary,add 3mLof a 1in 15solution of sodium nitrite in 2Nsulfuric acid.Add ammonium hydroxide dropwise:a yellow precipitate is formed and it dissolves upon the addition of sodium hydroxide solution (1in 5).
D:
To 1mLof a solution (1in 100)add 1mLof phosphomolybdic acid solution (1in 100):a pale yellow to white precipitate is formed.
pHá791ñ:
between 4.5and 6.0,in a solution (1in 100).
Loss on drying á731ñ—
Dry it at 105 for 1hour:it loses not more than 0.5%of its weight.
for 1hour:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.2%.
Heavy metals,Method IIá231ñ:
0.002%.
Related compounds—
To 10mg,accurately weighed in a suitable vial,add 1mLof N-trimethylsilylimidazole,and heat at 65 for 10minutes.Add 5mLof isooctane,wash with one 3-mLportion of water,and allow the layers to separate.Inject a 2-µLportion of the isooctane solution into a gas chromatograph equipped with a 0.3-cm ×2.0-m glass column packed with packing S1Acontaining 3%liquid phase G2and a flame-ionization detector.The column temperature is maintained at 215
for 10minutes.Add 5mLof isooctane,wash with one 3-mLportion of water,and allow the layers to separate.Inject a 2-µLportion of the isooctane solution into a gas chromatograph equipped with a 0.3-cm ×2.0-m glass column packed with packing S1Acontaining 3%liquid phase G2and a flame-ionization detector.The column temperature is maintained at 215 ,and the injection port and detector are maintained at 250
,and the injection port and detector are maintained at 250 .The carrier gas is nitrogen,flowing at the rate of 25mLper minute.Adjust the instrument to provide full-scale response for the major component.Inject a second 2-µLportion of the isooctane solution with the attenuator adjusted to an 8-fold increase in sensitivity,and record the chromatogram from 0.5to 1.5relative to the retention time of the major peak.Measure the area of all minor peaks,and correct for differences in sensitivity settings.Calculate the percentage of related compounds present taken by the formula:
.The carrier gas is nitrogen,flowing at the rate of 25mLper minute.Adjust the instrument to provide full-scale response for the major component.Inject a second 2-µLportion of the isooctane solution with the attenuator adjusted to an 8-fold increase in sensitivity,and record the chromatogram from 0.5to 1.5relative to the retention time of the major peak.Measure the area of all minor peaks,and correct for differences in sensitivity settings.Calculate the percentage of related compounds present taken by the formula:
100A/B,
in which Ais the sum of the corrected area peaks for all minor peaks,and Bis the sum of the corrected area peaks for the major and minor peaks.Not more than 2.0%is found.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Assay—
Transfer about 50mg of Isoxsuprine Hydrochloride,accurately weighed,to a 1000-mLvolumetric flask,add water to volume,and mix.Concomitantly determine the absorbances of this solution and of a Standard solution of USP Isoxsuprine Hydrochloride RSin the same medium having a known concentration of about 50µg per mLin 1-cm cells at the wavelengths of maximum absorbance at about 269and 300nm,with a suitable spectrophotometer,using water as the blank.Calculate the quantity,in mg,of C18H23NO3·HCl in the Isoxsuprine Hydrochloride taken by the formula:
C(AU269-AU300)/(AS269-AS300),
in which Cis the concentration,in µg per mL,of USP Isoxsuprine Hydrochloride RSin the Standard solution,and the parenthetic expressions are the differences in the absorbances of the two solutions at the wavelengths indicated by the subscripts,for the assay solution (U)and the Standard solution (S),respectively.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 1090
Phone Number:1-301-816-8305