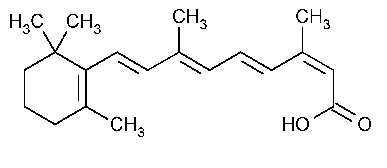
Isotretinoin
Retinoic acid,13-cis-.
3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)2-cis-4-trans-6-trans-8-trans-nonatetraenoic acid [4759-48-2].
»Isotretinoin contains not less than 98.0percent and not more than 102.0percent of C20H28O2,calculated on the dried basis.
Caution—Isotretinoin is teratogenic.Avoid inhalation and skin contact.
Packaging and storage—
Preserve in tight containers,under an atmosphere of an inert gas,protected from light.
USP Reference standards á11ñ—
USP Isotretinoin RS.USP Tretinoin RS.[NOTE—Avoid exposure to strong light,and use low-actinic glassware in the performance of the following procedures.]
Identification—
A:
Infrared Absorption á197Mñ.
B:
Ultraviolet Absorption á197Uñ—
Solution:
4µg per mL.
Medium:
acidified isopropyl alcohol (prepared by diluting 1mLof 0.01Nhydrochloric acid with isopropyl alcohol to 1000mL).Absorptivities at 354nm do not differ by more than 3.0%.
Loss on drying á731ñ—
Dry it in vacuum at room temperature for 16hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.002%.
Limit of tretinoin—
Mobile phase—
Prepare a suitable filtered and degassed mixture of isooctane,isopropyl alcohol,and glacial acetic acid (99.65:0.25:0.1).
Standard stock solution—
Dissolve an accurately weighed quantity of USP Tretinoin RSin a minimum quantity of methylene chloride,add an amount of isooctane to obtain a solution having a known concentration of about 250µg per mL,and mix.
Standard solution—
Pipet 1mLof Standard stock solutioninto a 100-mLvolumetric flask,add isooctane to volume,and mix.
Test solution—
Transfer about 25mg of Isotretinoin,accurately weighed,to a 100-mLvolumetric flask,dissolve in a minimum quantity of methylene chloride,add isooctane to volume,and mix.
System suitability stock solution—
Dissolve a quantity of USP Isotretinoin RSin a minimum amount of methylene chloride,add an amount of isooctane to obtain an isotretinoin concentration of about 250µg per mL,and mix.
System suitability solution—
Pipet 1mLof Standard stock solutioninto a 100-mLvolumetric flask,add System suitability stock solutionto volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 352-nm detector and a 4.0-mm ×25-cm column containing 5-µm packing L3.The flow rate is about 1mLper minute.Chromatograph about 20µLof the System suitability solution,and record the peak responses as directed for Procedure:the relative retention times for isotretinoin and tretinoin are about 0.84and 1.00,respectively;the resolution,R,between isotretinoin and tretinoin is not less than 2.0;and the relative standard deviation determined from the tretinoin peak response in replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the percentage of tretinoin by the formula:
10(C/W)(rU/rS),
in which Cis the concentration,in µg per mL,of USP Tretinoin RSin the Standard solution;Wis the weight,in mg,of Isotretinoin taken;and rUand rSare the tretinoin peak responses obtained from the Test solutionand the Standard solution,respectively.The content of tretinoin is not more than 1.0%.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent:
dimethylacetamide.
Assay—
Dissolve about 240mg of Isotretinoin,accurately weighed,in 50mLof dimethylformamide,add 3drops of a solution of thymol blue in dimethylformamide (1in 100),and titrate with 0.1Nsodium methoxide VSto a greenish endpoint.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nsodium methoxide is equivalent to 30.04mg of C20H28O2.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(PA6)Pharmaceutical Analysis 6
USP28–NF23Page 1088
Phone Number:1-301-816-8389