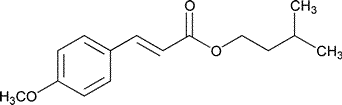
Amiloxate
»Amiloxate contains not less than 95.0percent and not more than 105.0percent of C15H20O3.
Packaging and storage—
Preserve in tight containers.
USP Reference standards á11ñ—
USP Amiloxate RS.
Identification—
B:
Ultraviolet Absorption á197Uñ—
Solution:
5.0µg per mL.
Medium:
alcohol.
Absorptivities,calculated on the as-is basis,do not differ by more than 3.0%.
Specific gravity á841ñ:
between 1.037and 1.041.
Refractive index á831ñ:
between 1.556and 1.560at 20 .
.
Acidity—
Transfer 50mLof alcohol to a suitable container,add 1mLof phenolphthalein TS,and add sufficient 0.1Nsodium hydroxide to obtain a persistent pink color.Transfer 50mLof this solution to a suitable container,add about 5.0mLof Amiloxate,accurately measured,mix,and titrate with 0.1Nsodium hydroxide:not more than 0.2mLof titrant per mLof Amiloxate is required for neutralization.
Chromatographic purity—
Test solution—
Use the Assay preparation.
Chromatographic system—
Proceed as directed in the Assay.To evaluate the system suitability requirements,use the Standard preparationprepared as directed in the Assay.
Procedure—
Inject a volume (about 1µL)of the Test solutioninto the chromatograph,record the chromatogram,and measure all of the peak responses.Calculate the percentage of each impurity in the portion of Amiloxate taken by the formula:
100(ri/rs),
in which riis the peak response for each impurity;and rsis the sum of the responses of all the peaks:not more than 0.1%of any individual impurity is found;and not more than 2.0%of total impurities is found.
Assay—
Standard preparation—
Dissolve an accurately weighed quantity of USP Amiloxate RSin tert-butyl methyl ether,and dilute quantitatively,and stepwise if necessary,with tert-butyl methyl ether to obtain a solution having a known concentration of about 20.0mg per mL.
Assay preparation—
Transfer about 2.0g of Amiloxate,accurately weighed,to a 100-mLvolumetric flask,dilute with acetone to volume,and mix.
Chromatographic system (see Chromatography á621ñ)—
The gas chromatograph is equipped with a flame-ionization detector and a 0.32-mm ×25-m column coated with a 0.1-µm film of G1.The carrier gas is helium,flowing at a rate of about 6mLper minute.The chromatograph is programmed as follows.Initially the temperature of the column is equilibrated at 60 ,then the temperature is increased at a rate of 8
,then the temperature is increased at a rate of 8 per minute to 240
per minute to 240 ,and maintained at 240
,and maintained at 240 for 10minutes.The injection port temperature is maintained at 240
for 10minutes.The injection port temperature is maintained at 240 ,and the detector temperature is maintained at 260
,and the detector temperature is maintained at 260 .Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the resolution,R,between the amiloxate peak and any other peak is not less than 1.0;and the relative standard deviation for replicate injections is not more than 2.0%.
.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the resolution,R,between the amiloxate peak and any other peak is not less than 1.0;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 1µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of C15H20O3in the portion of Amiloxate taken by the formula:
100C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Amiloxate RSin the Standard preparation;and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(PA6)Pharmaceutical Analysis 6
USP28–NF23Page 119
Pharmacopeial Forum:Volume No.27(5)Page 3017
Phone Number:1-301-816-8389