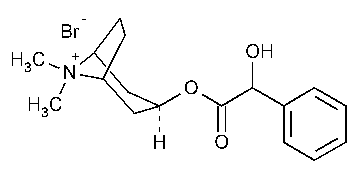
Homatropine Methylbromide
8-Azoniabicyclo[3.2.1]octane,3-(hydroxyphenylacetyl)oxy-8,8-dimethyl-,bromide,endo-.
3a-Hydroxy-8-methyl-1aH,5aH-tropanium bromide mandelate [80-49-9].
»Homatropine Methylbromide contains not less than 98.5percent and not more than 100.5percent of C17H24BrNO3,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
A:
Infrared Absorption á197Kñ.[NOTE—If differences are observed,dissolve the specimen and the Reference Standard separately in methanol,and recrystallize by adding dioxane to each solution.]
B:
Ultraviolet Absorption á197Uñ—
Solution:
1mg per mL.
Medium:
alcohol.
Absorptivities at 258nm,calculated on the dried basis,do not differ by more than 3.0%.
C:
Mercuric-potassium iodide TSproduces in a solution (1in 50)a white or slightly yellowish precipitate,but no precipitation is caused by solutions of alkali hydroxides or carbonates,even in concentrated solutions of the substance (distinction from most alkaloids).
D:
To a solution (1in 50),add ammonium reineckate TS:a red precipitate is formed.
E:
Asolution (1in 20)responds to the tests for Bromide á191ñ.
pHá791ñ:
between 4.5and 6.5,in a solution (1in 100).
Loss on drying á731ñ—
Dry it at 105 for 3hours:it loses not more than 0.5%of its weight.
for 3hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.2%.
Homatropine,atropine,and other solanaceous alkaloids—
To 1mLof a solution of it (1in 50),add a few drops of 6Nammonium hydroxide,shake the solution with 5mLof chloroform,and evaporate the separated chloroform layer on a steam bath to dryness.Warm the residue with 1.5mLof a solution made by dissolving 500mg of mercuric chloride in 25mLof a mixture of 5volumes of alcohol and 3volumes of water:no yellow or red color develops.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Dissolve about 700mg of Homatropine Methylbromide,accurately weighed,in a mixture of 50mLof glacial acetic acid and 10mLof mercuric acetate TS.Add 1drop of crystal violet TS,and titrate with 0.1Nperchloric acid VSto a blue-green endpoint.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 37.03mg of C17H24BrNO3.
Auxiliary Information—
Staff Liaison:Elena Gonikberg,Ph.D.,Scientist
Expert Committee:(PA4)Pharmaceutical Analysis 4
USP28–NF23Page 949
Phone Number:1-301-816-8251