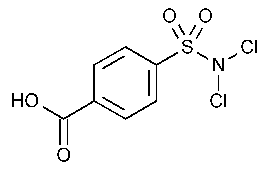
Halazone
Benzoic acid,4-[(dichloroamino)sulfonyl]-.
p-(Dichlorosulfamoyl)benzoic acid [80-13-7].
»Halazone contains not less than 91.5percent and not more than 100.5percent of C7H5Cl2NO4S,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
Add about 100mg to 5mLof sodium bromide solution (1in 10):bromine is liberated from the mixture.
Loss on drying á731ñ—
Dry it over phosphorus pentoxide for 4hours:it loses not more than 0.5%of its weight.
Readily carbonizable substances á271ñ—
Dissolve 100mg in 0.5mLof sulfuric acid TS:no blackening occurs,although some effervescence may take place.
Assay—
Add about 150mg of Halazone,accurately weighed,to 10mLof 2.5Nsodium hydroxide in a 250-mLiodine flask,stir well to dissolve,add 75mLof water,then promptly add 15mLof potassium iodide solution (1in 10),and mix.Acidify with 10mLof 6Nacetic acid,and immediately titrate the liberated iodine with 0.1Nsodium thiosulfate VS,adding 3mLof starch TSas the endpoint is approached.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nsodium thiosulfate is equivalent to 6.752mg of C7H5Cl2NO4S.
Auxiliary Information—
Staff Liaison:Radhakrishna S Tirumalai,Scientist
Expert Committee:(GTB)General Toxicology and Biocompatibility
USP28–NF23Page 935
Phone Number:1-301-816-8339