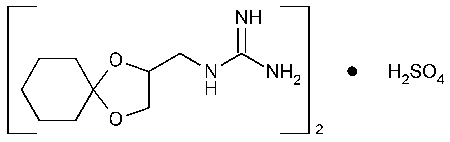
Guanadrel Sulfate
Guanidine (1,4-dioxaspiro[4.5]dec-2-ylmethyl)-,sulfate (2:1).
(1,4-Dioxaspiro[4.5]dec-2-ylmethyl)guanidine sulfate (2:1) [22195-34-2].
»Guanadrel Sulfate contains not less than 97.0percent and not more than 103.0percent of (C10H19N3O2)2·H2SO4,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
The IRabsorption spectrum of a mineral oil dispersion of it exhibits maxima only at the same wavelengths as that of a similar preparation of USP Guanadrel Sulfate RS.
Loss on drying á731ñ—
Dry it at room temperature at a pressure not exceeding 5mm of mercury for 16hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.5%.
Heavy metals,Method IIá231ñ:
0.002%.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Solvent—
Use a mixture of benzyl alcohol and water (100:7).
Assay—
Mobile phase—
Prepare a filtered and degassed mixture of 530mLof water and 470mLof methanol containing about 6.35g of dl-10-camphorsulfonic acid sodium salt and 0.8g of ammonium nitrate.Adjust with glacial acetic acid,if necessary,to a pHof between 5.0and 5.5.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Dissolve an accurately weighed quantity of USP Guanadrel Sulfate RSin Mobile phaseto obtain a solution having a known concentration of about 10mg per mL.
Assay preparation—
Transfer about 100mg of Guanadrel Sulfate,accurately weighed,to a container,add 10.0mLof Mobile phase,and mix.
Resolution solution—
Dissolve suitable quantities of USP Guanadrel Sulfate RSand ethylparaben in Mobile phaseto obtain a solution having known concentrations of about 10mg and 12mg,respectively,in each mL.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a refractive index detector and a 4-to 4.6-mm ×25-to 30-cm stainless steel column containing packing L1.The flow rate is about 1.5mLper minute.Chromatograph the Resolution solution,and record the peak responses as directed for Procedure:the resolution,R,between guanadrel and ethylparaben is not less than 1.6,and the relative retention times are about 0.8for guanadrel and 1.0for ethylparaben.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 2.5%.
Procedure—
Separately inject equal volumes (about 25µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of (C10H19N3O2)2·H2SO4in the portion of Guanadrel Sulfate taken by the formula:
10C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Guanadrel Sulfate RSin the Standard preparation;and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 931
Phone Number:1-301-816-8305