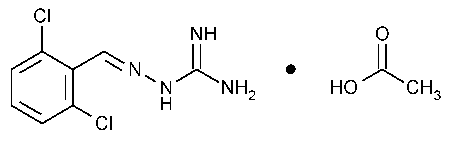
Guanabenz Acetate
Hydrazinecarboximidamide,2-[(2,6-dichlorophenyl)methylene]-,monoacetate.
[(2,6-Dichlorobenzylidene)amino]guanidine monoacetate [23256-50-0].
»Guanabenz Acetate contains not less than 98.0percent and not more than 101.5percent of the labeled amount of C10H12N4O2Cl2.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification,Infrared Absorption á197Kñ.
pHá791ñ:
between 5.5and 7.0,in a solution (7in 1000).
Loss on drying á731ñ—
Dry it in vacuum at 60 for 2hours:it loses not more than 1.0%of its weight.
for 2hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.2%.
Limit of 2,6-dichlorobenzaldehyde—
Internal standard solution 1—
Dissolve 100mg of p-chlorobenzaldehyde in 100mLof chloroform,and mix.
Internal standard solution 2—
Dilute 1.0mLof Internal standard solution 1to 10.0mLwith chloroform,and mix.
Standard solution—
Prepare a solution of 2,6-dichlorobenzaldehyde in chloroform containing 1.0mg per mL.
Standard preparation—
Transfer 4.0mLof Standard solutionand 1.0mLof Internal standard solution 1to a 10-mLvolumetric flask,dilute with chloroform to volume,and mix.
Test preparation—
Transfer 200mg of Guanabenz Acetate to a 30-mLglass-stoppered centrifuge tube.Add 10mLof 0.1Nhydrochloric acid,shake to dissolve,add 1.0mLof Internal standard solution 2,and shake.Centrifuge,and transfer a portion of the lower layer to a stoppered container.[NOTE—The lower layer must be removed within 10minutes of adding the acid to the centrifuge tube.]
Chromatographic system
(see Chromatography á621ñ)—The gas chromatograph is equipped with a flame-ionization detector and a 1.8-m ×3-mm column packed with 20%phase G1on 80-to 100-mesh support S1A.The column is maintained at a temperature of about 190 ,the injection port at about 225
,the injection port at about 225 ,and the detector at about 250
,and the detector at about 250 .Nitrogen is used as the carrier gas at a flow rate of about 30mLper minute.
.Nitrogen is used as the carrier gas at a flow rate of about 30mLper minute.
Procedure—
Separately inject 2-µLportions of the Standard preparationand the Test preparation,successively,into the gas chromatograph.The resolution between 2,6-dichlorobenzaldehyde and p-chlorobenzaldehyde is not less than 3.0,and the relative retention time for p-chlorobenzaldehyde is 0.5and for 2,6-dichlorobenzaldehyde is 1.0.The relative peak response ratio obtained from the Test preparationdoes not exceed that obtained from the Standard preparation(0.2%).
Chromatographic purity—
Methanolic formic acid—
Prepare a mixture of formic acid and methanol (1in 2000).
Aminoguanidine bicarbonate solution—
Transfer 100mg of aminoguanidine bicarbonate to a test tube,add 0.05mLof formic acid,and warm gently to effect solution.Quantitatively transfer the contents of the test tube to a 10-mLvolumetric flask,dilute with methanol to volume,and mix.
Standard solution A—
Transfer 10mg of USP Guanabenz Acetate RSto a 100-mLvolumetric flask,and dissolve in 50mLof Methanolic formic acid.Add 1.0mLof the Aminoguanidine bicarbonate solution,dilute with Methanolic formic acidto volume,and mix.
Standard solution B—
Transfer 5.0mLof Standard solution Ato a 10-mLvolumetric flask,dilute with Methanolic formic acidto volume,and mix.
Standard solution C—
Transfer 2.0mLof Standard solution Ato a 10-mLvolumetric flask,dilute with Methanolic formic acidto volume,and mix.
Test solution—
Prepare a solution of guanabenz acetate containing 10mg per mLin Methanolic formic acid.
Procedure—
Prepare a chromatographic chamber containing a mixture of chloroform,methanol,and ammonium hydroxide (60:40:1)as the developing solvent,and allow it to equilibrate for at least 30minutes before use.Prewash a plate coated with a 0.25-mm layer of chromatographic silica gel mixture (see Chromatography á621ñ)by placing it in the chromatographic chamber,allowing the solvent front to rise to the top of the plate,drying it in air and activating it by heating at 105 for 20minutes.Within 30minutes after preparation,separately apply 10-µLportions of Standard solutions A,B,and C,the Test solution,and Methanolic formic acid.Allow the spots to dry,and place the plate in the chromatographic chamber.When the solvent has moved about three-fourths of the length of the plate,remove the plate and allow it to air-dry for about 30minutes.Examine the plate under short-wavelength UVlight.Estimate the amount of any secondary spots (other than any secondary spot with the same RFas the Methanolic formic acid)observed in the chromatogram of the Test solutionby comparison with the Standard solutions.Place the plate in a chamber saturated with iodine vapors for about 10minutes.Remove and examine the plate.Estimate the amount of any spot in the chromatogram of the Test solutionthat has an RFcorresponding to the RFof the spot produced by the aminoguanidine bicarbonate by comparison with the Standard solutions.No individual secondary spot is greater in size or intensity than the spot produced by Standard solution B(0.5%),and the total of any such spots observed is not more than 1%.
for 20minutes.Within 30minutes after preparation,separately apply 10-µLportions of Standard solutions A,B,and C,the Test solution,and Methanolic formic acid.Allow the spots to dry,and place the plate in the chromatographic chamber.When the solvent has moved about three-fourths of the length of the plate,remove the plate and allow it to air-dry for about 30minutes.Examine the plate under short-wavelength UVlight.Estimate the amount of any secondary spots (other than any secondary spot with the same RFas the Methanolic formic acid)observed in the chromatogram of the Test solutionby comparison with the Standard solutions.Place the plate in a chamber saturated with iodine vapors for about 10minutes.Remove and examine the plate.Estimate the amount of any spot in the chromatogram of the Test solutionthat has an RFcorresponding to the RFof the spot produced by the aminoguanidine bicarbonate by comparison with the Standard solutions.No individual secondary spot is greater in size or intensity than the spot produced by Standard solution B(0.5%),and the total of any such spots observed is not more than 1%.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Assay—
Dissolve about 200mg of Guanabenz Acetate,accurately weighed,in 50mLof glacial acetic acid.Titrate with 0.1Nperchloric acid VS,determining the endpoint potentiometrically.Perform a blank determination (see Titrimetry á541ñ),and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 29.12mg of C10H12N4O2Cl2.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 929
Phone Number:1-301-816-8305