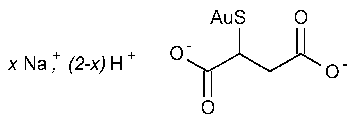
Gold Sodium Thiomalate
Butanedioic acid,mercapto-,monogold(1+)sodium salt.
Mercaptosuccinic acid,monogold(1+)sodium salt [12244-57-4].
»Gold Sodium Thiomalate is a mixture of the mono-and di-sodium salts of gold thiomalic acid.It contains not less than 44.8percent and not more than 49.6percent of Au.It contains not less than 49.0percent and not more than 52.5percent of Au on a dry,alcohol-and glycerin-free basis.
Packaging and storage—
Preserve in tight,light-resistant containers.Store at 25 ,excursions permitted between 15
,excursions permitted between 15 and 30
and 30 .
.
Identification—
A:
To 2mLof a solution (1in 10)add 1mLof calcium nitrate solution (1in 10):a white precipitate is formed,and it dissolves in 2Nnitric acid and reappears upon the addition of ammonium acetate TS.
B:
To 2mLof a solution (1in 10)add 4mLof silver nitrate TS:a yellowish precipitate is formed,and it dissolves completely in an excess of 6Nammonium hydroxide.
C:
To 2mLof a solution (1in 10)add 1mLof 6Nammonium hydroxide and 1mLof 30percent hydrogen peroxide,evaporate in a porcelain dish,and ignite.Add 20mLof water to the ignited residue,and filter:particles of gold remain on the filter.Separate portions of the filtrate meet the requirements of the tests forSodium á191ñand forSulfate á191ñ.
pHá791ñ:
between 5.8and 6.5,in a solution (1in 10).
Loss on drying á731ñ—
Dry it at 60 and at a pressure not exceeding 5mm of mercury for 2hours:it loses not more than 8.0%of its weight.
and at a pressure not exceeding 5mm of mercury for 2hours:it loses not more than 8.0%of its weight.
Limit of alcohol—
Standard solution—
Transfer 50mg of dehydrated alcohol to a 200-mLvolumetric flask,dilute with water to volume,and mix.Transfer 5.0mLto a 50-mLvolumetric flask,and dilute with water to volume.This solution contains about 0.025mg of alcohol per mL.
Test solution—
Transfer about 50mg of Gold Sodium Thiomalate,accurately weighed,to a 10-mLvolumetric flask,and dilute with water to volume.
Chromatographic system(see Chromatography á621ñ)—
The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm ×30-m fused-silica capillary column coated with a 3.0-µm film of phase G43.The column temperature is maintained at 40 for 8.5minutes,then the temperature is increased at 30
for 8.5minutes,then the temperature is increased at 30 per minute to 240
per minute to 240 .The total chromatographic time is about 15minutes.The injection port and detector block temperatures are maintained at 150
.The total chromatographic time is about 15minutes.The injection port and detector block temperatures are maintained at 150 .The carrier gas is helium,flowing at a rate of about 2mLper minute,and the split flow rate is about 20mLper minute.Chromatograph theStandard solution,and record the peak responses as directed forProcedure:the relative standard deviation for replicate injections is not more than 4.6%.
.The carrier gas is helium,flowing at a rate of about 2mLper minute,and the split flow rate is about 20mLper minute.Chromatograph theStandard solution,and record the peak responses as directed forProcedure:the relative standard deviation for replicate injections is not more than 4.6%.
Procedure—
Separately inject equal volumes (about 1µL)of theStandard solutionand theTest solution into the chromatograph,record the chromatograms,and measure the peak responses.Calculate the percentage of alcohol (C2H5OH)in the portion of Gold Sodium Thiomalate taken by the formula:
100(Ca/Cb)(rU/rS),
in whichCais the concentration,in mg per mL,of C2H5OHin theStandard solution;Cbis the concentration,in mg per mL,of Gold Sodium Thiomalate in theTest solution;andrUandrSare the alcohol peak responses obtained from theTest solutionand theStandard solution,respectively.Not more than 4.0%is found.
Limit of glycerin—
[NOTE—This procedure is based on the absorption characteristics of a sodium–copper–glycerin complex.The stability of this complex prepared as directed herein is such that all measurements are to be taken within 1hour.Thoroughly rinse all glassware used in this procedure with water to avoid large blank errors.]
Sodium hydroxide solution—
Dissolve 23.6g of sodium hydroxide in water to obtain 100mLof solution.
Cupric chloride solution—
Dissolve 3.8g of cupric chloride in water to obtain 100mLof solution.
Glycerin standard solutions—
Dissolve an accurately weighed quantity of glycerin in water to obtain a solution having a known concentration of about 8mg per mL.Pipet 1.0,2.0,and 3.0mLof this solution into a series of 10-mLvolumetric flasks,followed by 4.0,3.0,and 2.0mLof water,respectively.
Reagent blank—
Pipet 5.0mLof water into a 10-mLvolumetric flask.
Test solution—
Dissolve about 400mg Gold Sodium Thiomalate,accurately weighed,in 5.0mLof water in a 10-mLvolumetric flask.
Procedure—
To each of theGlycerin standard solutions,theReagent blank,and theTest solutionadd 1.0mLofSodium hydroxide solution,and mix.With vigorous shaking,and in increments of 0.1mL,addCupric chloride solution,checking for turbidity after each addition.After the solutions turn slightly turbid,add an excess of 0.1mLofCupric chloride solution,insert the stopper,and shake for 1minute.Dilute with water to volume,and mix.Centrifuge the solutions in tapered,graduated 15-mLcentrifuge tubes.The presence of 1mm to 4mm of copper hydroxide precipitate is observed.Using a suitable spectrophotometer equipped with 1-cm cells,and using water as a reference,measure the absorbance of the clear supernatant at a wavelength of 635nm.Subtract the absorbance value of theReagent blank,which is 0.040or less,from the absorbance values of theGlycerin standard solutionsand theTest solution.Plot the corrected absorbance readings of theGlycerin standard solutionsagainst the corresponding weight of glycerin.From the standard curve so obtained,and the corrected absorbance of theTest solution,determine the weight of glycerin in the test specimen:not more than 5.5%is found.
Assay—
Dissolve about 600mg of Gold Sodium Thiomalate,accurately weighed,in water in a 25-mLvolumetric flask,dilute with water to volume,and mix.Pass the entire solution through a clean,dry 0.5-µm filter into a clean,dry receiver.Pipet 20.0mLof the filtrate into a 300-mL Kjeldahl flask,add 20mLof nitric acid,and mix.To this solution add 15mLof sulfuric acid slowly,with mixing.Heat over a low flame,gently at first,and then increase the heat until fumes of sulfur trioxide are evolved.Allow the flask and contents to cool to room temperature,add 30mLof water slowly,with mixing,and 20mLof hydrogen peroxide TS,again heat to fumes of sulfur trioxide,cool,and dilute with 30mLof water.Pass the mixture through an ignited,tared filtering crucible,wash with water,heat the crucible and contents over a low flame to dry the precipitate,and ignite at 650±50 to constant weight.The weight of the residue so obtained,multiplied by 1.25,is the weight of Au in the Gold Sodium Thiomalate taken.
to constant weight.The weight of the residue so obtained,multiplied by 1.25,is the weight of Au in the Gold Sodium Thiomalate taken.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA2)Pharmaceutical Analysis 2
USP28–NF23Page 915
Pharmacopeial Forum:Volume No.29(6)Page 1895
Phone Number:1-301-816-8139