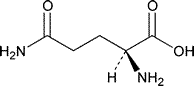
Glutamine
»Glutamine contains not less than 98.5percent and not more than 101.5percent of C5H10N2O3,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers,and store at controlled room temperature.
Identification,Infrared Absorption á197Kñ.
Specific rotation á781Sñ:
between +6.3 and +7.3
and +7.3 ,determined at 20
,determined at 20 .
.
Test solution—
In a suitable flask,accurately prepare a solution of about 40mg per mLin water at about 40 .Cool and dilute with water to volume before use.
.Cool and dilute with water to volume before use.
Loss on drying á731ñ—
Dry it at 105 for 3hours:it loses not more than 0.3%of its weight.
for 3hours:it loses not more than 0.3%of its weight.
Residue on ignition á281ñ:
not more than 0.3%.
Chloride á221ñ—
A0.7-g portion shows no more chloride than corresponds to 0.50mLof 0.020Nhydrochloric acid:not more than 0.05%is found.
Sulfate á221ñ—
A0.8-g portion shows no more sulfate than corresponds to 0.25mLof 0.020Nsulfuric acid:not more than 0.03%is found.
Iron á241ñ:
0.003%.
Heavy metals,Method Iá231ñ:
0.0015%.
Chromatographic purity—
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture.
Test solution:
10mg per mL,in water.
Standard solution—
Prepare a solution of USP Glutamine RSin water having a known concentration of about 0.05mg per mL.
Application volume:
5µL.
Developing solvent system:
a mixture of butyl alcohol,water,and glacial acetic acid (3:1:1).
Spray reagent—
Dissolve 0.2g of ninhydrin in 100mLof a mixture of butyl alcohol and 2Nacetic acid (95:5).
Procedure—
Proceed as directed for System Suitabilityunder Chromatography á621ñ,then dry the plate at 80 for 30minutes.Spray the plate with the Spray reagent,heat at 80
for 30minutes.Spray the plate with the Spray reagent,heat at 80 for 10minutes,and examine under white light:no secondary spot in the chromatogram obtained from the Test solutionis larger or more intense than the principal spot in the chromatogram obtained from the Standard solution(0.5%).
for 10minutes,and examine under white light:no secondary spot in the chromatogram obtained from the Test solutionis larger or more intense than the principal spot in the chromatogram obtained from the Standard solution(0.5%).
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Transfer about 150mg of Glutamine,accurately weighed,to a 125-mLflask,dissolve in 3mLof formic acid and 50mLof glacial acetic acid,and titrate with 0.1Nperchloric acid VS,determining the endpoint potentiometrically.Perform a blank determination,and make any necessary correction (see Titrimetry á541ñ).Each mLof 0.1Nperchloric acid is equivalent to 14.615mg of C5H10N2O3.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(DSN)Dietary Supplements:Non-Botanicals
USP28–NF23Page 909
Phone Number:1-301-816-8389