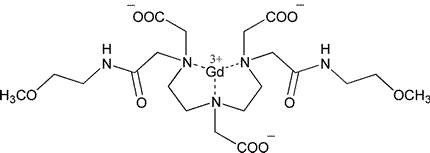
Gadoversetamide
(Gadoversetamide)[8,11-bis(carboxymethyl)-14-[2-[(2-methoxyethyl)amino]-2-oxoethyl]-6-oxo-2-oxa-5,8,11,14-tetraazahexadecan-16-oato(3-)],gadolinium.
[N,N-Bis[2-[(carboxymethyl)[[(2-methoxyethyl)carbamoyl]methyl]amino]ethyl]glycinato(3-)]gadolinium [131069-91-5].
»Gadoversetamide contains not less than 97.0percent and not more than 102.0percent of C20H34GdN5O10,calculated on an anhydrous basis.
Packaging and storage—
Preserve in tight,light-resistant containers,and store at controlled room temperature.
USP Reference standards á11ñ—
USP Endotoxin RS.USP Gadodiamide Related Compound B RS.USP Gadoversetamide RS.USP Gadoversetamide Related Compound A RS.
Identification—
A:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation,as obtained in the Assay.
B:
The lanthanide selectivity test detects gadolinium (III)in 0.1Nnitric acid with arsenazo (III).Prepare 1.5×10–4Marsenazo (III)solution by dissolving 30mg of arsenazo (III)and 160mg of urea in 100mLwater,adding 1.6mLof nitric acid,and diluting with water to 250mL.Add 10mg of Gadoversetamide to 1.0mLof the 1.5×10–4Marsenazo (III)solution,and mix.The color changes from a wine red to green-blue,indicating the presence of gadolinium.
Bacterial endotoxins á85ñ:
not more than 15USP Endotoxin Units per g of gadoversetamide.
Water,Method Ia á921ñ:
not more than 10.0%(w/w),a solvent mixture of methanol and formamide (9:1)being used.
Limits of free gadolinium (III)and total chelatable material—
MESbuffer—
Dissolve 97.6g of 2-morpholinoethanesulfonic acid (MES)in about 950mLof water,and mix.Adjust with 20%sodium hydroxide to a pHof 6,dilute with water to 1000mL,and mix.
Edetate titrant:
0.002Medetate disodium VS.
0.003M Gadolinium (III)titrant—
Transfer 0.790g of gadolinium chloride to a 1000-mLvolumetric flask,dilute with water to volume,and mix.
Test solution—
Transfer about 5g of Gadoversetamide,accurately weighed,into a 250-mLflask,and add 20mLof water and 2mLof hydrochloric acid.Stir,and heat to boiling.Rinse the sides of the flask with water.Add 50mLof MESbufferand 100to 150µLof xylenol orange TSto impart a light yellow color.Heat to boiling,adjust with ammonium hydroxide to a pHof 6,and continue boiling for 2minutes.If the solution is yellow,proceed as directed under Uncomplexed chelatable material.If the color is red-violet,proceed as directed under Free gadolinium (III).
Uncomplexed chelatable material—
Continue boiling,and titrate with 0.003M Gadolinium (III)titrantto a red-violet endpoint that undergoes no further color change upon addition of more titrant.Record the volume of titrant used to reach the endpoint.
Free gadolinium (III)—
Continue boiling and titrate with Edetate titrantto a yellow or yellow-orange endpoint that undergoes no further color change upon addition of more titrant.Record the volume of titrant used to reach the endpoint.
Calculations—
If the Test solution was titrated with Edetate titrant,calculate the percentage ofFree gadolinium (III)in the portion of Gadoversetamide taken by the formula:
(66.18/W)(VEU)(ME),
in which Wis the weight,in g,of Gadoversetamide taken;VEUis the volume,in mL,of Edetate titrantused to titrate the Test solution;andMEis the molarity of the Edetate titrant.If the sample was titrated with 0.003M Gadolinium (III)titrant,calculate the percentage of Uncomplexed chelatable materialin the portion of Gadoversetamide taken by the formula:
(66.18/W)(VGU)(MG),
in which Wis as defined herein;VGUis the volume,in mL,of 0.003M Gadolinium (III)titrantused to titrate theTest solution;andMGis the molarity of the 0.003M Gadolinium (III)titrant.Not more than 0.05%of free gadolinium IIIand not more than 0.1%of uncomplexed chelatable material,both calculated on the anydrous basis,are found.
Limit of 2-methoxyethylamine—
Mobile phase—
Add 2mLof 5Mphosphoric acid to 550mLof water,mix,and adjust with 10%(w/w)ammonium hydroxide to a pHof 5.0.Add 450mLof acetonitrile,mix,filter,and degas.
0.4M Borate buffer—
Add 12.4g of boric acid to 300mLof water,and swirl to suspend.Add 100mLof 1Npotassium hydroxide,and mix.Adjust with about 60mLof 1Npotassium hydroxide to a pHof 10.0,dilute with water to 500mL,and mix.Test the pH,and adjust if necessary.
o-Phthalaldehyde reagent—
Dissolve 25mg of o-phthalaldehyde in 0.75mLof methanol,add 25mLof 0.4M Borate bufferhaving a pHof 10.0and 25µLof 2-mercaptoethanol,and mix.[NOTE—Protect from light.Discard after 3days.]
Standard solutions—
Prepare aqueous solutions of 2-methoxyethylamine having known concentrations of about 1,20,and 50µg per mL,respectively.Derivatize by adding an equal volume of o-Phthalaldehyde reagentto each solution immediately before injection.
Test solution—
Transfer about 250mg of Gadoversetamide,accurately weighed,to a 5-mLvolumetric flask,and dissolve in and dilute with water to volume.Derivatize the solution by combining equal volumes of o-Phthalaldehyde reagentand Test solutionimmediately before injection.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 335-nm detector and a 250-mm ×4.6-mm column that contains 5-µm packing L1.The flow rate is about 1mLper minute.Chromatograph the Standard solutions,and record the chromatograms as directed for Procedure:the relative retention times of o-phthalaldehyde and 2-methoxyethylamine are about 0.6and 1.0,respectively.Plot the concentration of 2-methoxyethylamine in each Standard solutionversus its peak area,and perform a regression analysis to obtain a slope and an intercept.The correlation coefficient,r,is not less than 0.995,and the relative standard deviation for replicate injections of the 50µg per mLStandard solutionis not more than 5%.
Procedure—
Separately inject equal volumes (about 50µL)of the Test solutionand the Standard solutionsinto the chromatograph,record the chromatograms,and measure the peak responses.Determine the concentration,in µg per mL,of 2-methoxyethylamine in the Test solutionfrom the standard response line.Calculate the percentage of 2-methoxyethylamine by the formula:
0.5C/W,
in which Cis the concentration,in µg per mL,of 2-methoxyethylamine obtained from the Standard response line;and Wis the weight,in mg,of Gadoversetamide taken.Not more than 0.10%(w/w)of 2-methoxyethylamine is present,calculated on the anhydrous basis.
Limit of residual solvents—
Internal standard solution—
Dilute butyl alcohol with water (3:5000).
Standard solutions—
To four separate 5-mLvolumetric flasks,transfer the following designated compositions:
Dilute each flask with water to volume,and mix.The resulting Standard solutionscontain about 5,20,50,and 100µg of isopropyl alcohol and acetonitrile per mL.
| Flask | Isopropyl alcohol |
Acetonitrile | Internal standard |
| 1 | 25µg | 25µg | 1.0mL |
| 2 | 100µg | 100µg | 1.0mL |
| 3 | 250µg | 250µg | 1.0mL |
| 4 | 500µg | 500µg | 1.0mL |
Test solution—
Transfer about 500mg of Gadoversetamide,accurately weighed,to a 5-mLvolumetric flask.Add 1.0mLof Internal standard solution,dissolve in and dilute with water to volume,and mix.
Chromatographic system (see Chromatography á621ñ)—
The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm ×30-m capillary column with a 1.0-µm thickness of phase G35.Helium is used as the carrier gas,at a flow rate of about 5mLper minute.The column temperature is maintained at 35 for 5minutes,then increased at a rate of 15
for 5minutes,then increased at a rate of 15 per minute to 110
per minute to 110 .The injection port temperature is maintained at 150
.The injection port temperature is maintained at 150 ,and the detector temperature is maintained at 300
,and the detector temperature is maintained at 300 .Chromatograph the Standard solutions,and record the peak area ratios as directed for Procedure:the relative retention times are about 0.5for isopropyl alcohol,0.7for acetonitrile,and 1.0for butyl alcohol.Plot the concentration for each standard versus its peak area ratio,and perform a regression analysis.The correlation coefficient,r,is not less than 0.995for each analyte;and the relative standard deviation for replicate injections of the 100µg per mLStandard solutionis not more than 5%.
.Chromatograph the Standard solutions,and record the peak area ratios as directed for Procedure:the relative retention times are about 0.5for isopropyl alcohol,0.7for acetonitrile,and 1.0for butyl alcohol.Plot the concentration for each standard versus its peak area ratio,and perform a regression analysis.The correlation coefficient,r,is not less than 0.995for each analyte;and the relative standard deviation for replicate injections of the 100µg per mLStandard solutionis not more than 5%.
Procedure—
Separately inject equal volumes (about 2µL)of the Test solutionand the Standard solutionsinto the chromatograph,record the chromatograms,and measure the peak area ratios of the standard peak to the internal standard peak.Determine the concentration of isopropyl alcohol and acetonitrile from the respective standard response lines.Calculate the percentage (w/w)of each solvent in the portion of Gadoversetamide taken by the formula:
0.5C/W,
in which Cis the concentration,in µg per mL(obtained from the respective standard response line)of isopropyl alcohol and acetonitrile in the Test solution;and Wis the weight (anhydrous),in mg,of the portion of Gadoversetamide taken:not more than 0.1%(w/w)of isopropyl alcohol is found;and not more than 0.025%(w/w)of acetonitrile is found.The total residual solvent content (sum of the %w/w isopropyl alcohol and the %w/w acetonitrile)does not exceed 0.1%w/w.
Related compounds—
Solution A—
Dissolve 2.06g of monobasic potassium phosphate and 18.6mLof 20%w/w tetraethylammonium hydroxide in 950mLof water.Adjust with phosphoric acid to a pHof 7,dilute with water to make 1000mL,mix,filter,and degas.
Solution B—
Prepare a filtered and degassed mixture of Solution Aand acetonitrile (475:25).
Mobile phase—
Use a mixture of Solution Aand Solution Bas directed for Chromatographic system.Make adjustments if necessary (see System Suitability under Chromatography á621ñ).
Standard stock solution—
Transfer about 50mg each of USP Gadoversetamide Related Compound A RSand USP Gadodiamide Related Compound B RS,accurately weighed,to a 50-mLvolumetric flask,dissolve in and dilute with water to volume,and mix.[NOTE—The solution may be stored for a week.]
Standard solutions—
Prepare aqueous solutions of diluted Standard stock solutioncontaining about 25,150,and 250µg of each Reference Standard per mL.
Test solution—
Transfer about 250mg of Gadoversetamide,accurately weighed,to a 10-mLvolumetric flask,dissolve in and dilute with water to volume,and mix.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 205-nm detector and a metal-free 4.6-×150-mm column that contains 5-µm packing L1.The flow rate is 1mLper minute.The chromatograph is programmed to pump a mixture of Solution Ato Solution B(97:3).The column temperature is maintained at about 25 .The relative retention times are about 0.6for gadodiamide related compound Band 0.7for gadoversetamide related compound A;the resolution,R,between gadodiamide related compound Band gadoversetamide related compound Ais not less than 1.0;and the relative standard deviation for replicate injections is not more than 5%for the 250µg per mLStandard solution.
.The relative retention times are about 0.6for gadodiamide related compound Band 0.7for gadoversetamide related compound A;the resolution,R,between gadodiamide related compound Band gadoversetamide related compound Ais not less than 1.0;and the relative standard deviation for replicate injections is not more than 5%for the 250µg per mLStandard solution.
Procedure—
Separately inject equal volumes (about 50µL)of the Test solutionand the Standard solutionsinto the chromatograph.Allow 1hour between injections to remove slow-eluting impurities.Determine the quantities,in µg per mL,of gadoversetamide related compound Aand gadodiamide related compound Bfrom the respective Standard response lines.Calculate the percentage of gadoversetamide related compound Ain the portion of Gadoversetamide taken by the formula:
100C/V,
in which Cis the concentration of gadoversetamide related compound A,in µg per mL,obtained from the Standard response line;and Vis the concentration of gadoversetamide,in µg per mL,in the Test solution.Not more than 1.0%(w/w)of gadoversetamide related compound Ais found,calculated on the anhydrous basis.Calculate the percentage of gadodiamide related compound Bin the portion of Gadoversetamide taken by the following formula:
92.2C/V,
in which Cis the concentration of gadodiamide related compound B,in µg per mL,obtained from the Standard response line;and Vis as described herein.Not more than 0.5%(w/w)of gadodiamide related compound Bis found,calculated on the anhydrous basis.
Assay—
Mobile phase—
Dissolve 1.5g of boric acid in about 950mLof water,and mix.Adjust with ammonium hydroxide to a pHof 6.8,add 15mLof acetonitrile,dilute with water to make 1000mL,mix,filter,and degas.
Standard preparations—
Prepare solutions of USP Gadoversetamide RSin Mobile phasehaving known concentrations of about 1.2,1.0,and 0.8mg per mL.
Assay preparation—
Transfer about 100mg of Gadoversetamide,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with Mobile phaseto volume,and mix.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 205-nm detector and a metal-free 4.6-×250-mm column that contains 5-µm packing L1.The column temperature is maintained at about 50 .The flow rate is about 1mLper minute.Chromatograph the Standard preparations,and record the peak responses as directed for Procedure.Plot the concentration of each Standard versus its peak area and perform a regression analysis to obtain a slope and intercept for the Standard response line.The correlation coefficient,r,is not less than 0.995;and the relative standard deviation for replicate injections of the 1.0mg per mLStandard preparationis not more than 2%.
.The flow rate is about 1mLper minute.Chromatograph the Standard preparations,and record the peak responses as directed for Procedure.Plot the concentration of each Standard versus its peak area and perform a regression analysis to obtain a slope and intercept for the Standard response line.The correlation coefficient,r,is not less than 0.995;and the relative standard deviation for replicate injections of the 1.0mg per mLStandard preparationis not more than 2%.
Procedure—
Separately inject equal volumes (about 10µL)of theAssay preparationand Standard preparationsinto the chromatograph,record the chromatograms,and measure the area of the gadoversetamide peak.Determine the quantity,in mg per mL,of gadoversetamide from the Standard response line.Calculate the quantity,in %(w/w),of C20H34GdN5O10in the portion of Gadoversetamide taken by the formula:
10,000C/W,
in which Cis the concentration,in mg per mL,obtained from the Standard response line;and Wis the weight (anhydrous),in mg,of the portion of Gadoversetamide taken to prepare the Assay preparation.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(RMI)Radiopharmaceuticals and Medical Imaging Agents
USP28–NF23Page 885
Pharmacopeial Forum:Volume No.29(5)Page 1497
Phone Number:1-301-816-8305