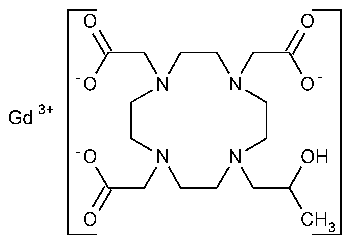
Gadoteridol
Gadolinium,[10-(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triacetato(3-)-N1,N4,N7,N10,O1,O4,O7,O10]-.
(±)-[10-(2-Hydroxypropyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triacetato(3-)]gadolinium [120066-54-8].
»Gadoteridol contains not less than 97.0percent and not more than 101.0percent of C17H29GdN4O7,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight,light-resistant containers,and store at controlled room temperature.
USP Reference standards á11ñ—
USP Gadoteridol RS.USP Gadoteridol Related Compound A RS.USP Gadoteridol Related Compound B RS.USP Gadoteridol Related Compound C RS.
Identification—
Water,Method Iá921ñ:
not more than 15%.
Heavy metals,Method IIá231ñ:
not more than 0.001%.
Limit of gadoteridol related compound A—
Buffer solution—
Dissolve 0.60g of tromethamine and 3.72g of edetate disodium in 3Lof water,adjust with 5Nsodium hydroxide to a pHof 7.0,dilute with water to 4L,and mix.Filter,and chill between 5 and 8
and 8 .
.
Mobile phase—
Prepare a filtered and degassed mixture of Buffer solution,acetonitrile,and tetrahydrofuran (98.8:1.0:0.2).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Cupric acetate solution—
Transfer 3.99g of cupric acetate and 12.11g of tromethamine to a 2-Lvolumetric flask,dissolve in 1500mLof water,adjust with glacial acetic acid to a pHof 7.0,dilute with water to volume,and mix.Filter,and chill between 5 and 8
and 8 .
.
Standard stock solution—
Dissolve an accurately weighed quantity of USP Gadoteridol Related Compound A RSin Buffer solution,and dilute quantitatively and stepwise with Buffer solutionto obtain a solution having a known concentration of about 6µg per mL.
Standard solution—
Just prior to injection onto the column,dilute 1.0mLof the Standard stock solutionwith 1.0mLof Cupric acetate solutionto obtain a solution having a known concentration of about 3µg of USP Gadoteridol Related Compound A RSper mL.[NOTE—The copper–gadoteridol related compound Acomplex is stable for at least 2hours,if maintained at about 5 .]
.]
Test solution—
Transfer about 30mg of Gadoteridol,accurately weighed,to a test tube,add 1.0mLof Buffer solution,and mix on a vortex mixer to dissolve.Add 1.0mLof Cupric acetate solution,and mix.Immediately inject the solution into the chromatograph.[NOTE—The copper–gadoteridol related compound Acomplex is stable for at least 2hours,if maintained at about 5 .]
.]
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 280-nm detector and a 4.0-mm ×15-cm column that contains packing L21.The flow rate is about 2mLper minute.Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the relative retention times are about 1.0for gadoteridol related compound Aand 2.0for copper-edetate;the resolution,R,between gadoteridol related compound Aand copper-edetate is not less than 1.5;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 50µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the peak responses.Calculate the percentage of gadoteridol related compound Ain the portion of Gadoteridol taken by the formula:
100(C/W)(rU/rS),
in which Cis the concentration,in mg per mL,of USP Gadoteridol Related Compound A RSin the Standard stock solution;Wis the weight,in mg,of Gadoteridol taken to prepare the Test solution;and rUand rSare the gadoteridol related compound Apeak responses obtained from the Test solutionand the Standard solution,respectively:not more than 0.01%is found,calculated on the anhydrous basis.
Limit of free gadolinium (III)—
Buffer solution,Mobile phase,Diluent,Standard stock solution 1,Standard stock solution 2,Standard solution,and Chromatographic system—
Proceed as directed for Chromatographic purity,Test 1.
Test solution—
Transfer about 60mg of Gadoteridol,accurately weighed,to a vial,add 2.0mLof Diluent,and mix.Immediately place the vial in a bath maintained at about 5 .
.
Procedure—
Proceed as directed for Procedureunder Chromatographic purity,Test 1.Calculate the percentage of free gadolinium (III)in the portion of Gadoteridol taken by the formula:
200(157.25/334.38)(C/W)(rU/rS),
in which 157.25is the molecular weight of gadolinium and 334.38is the molecular weight of gadolinium acetate;Cis the concentration,in mg per mL,of gadolinium acetate,calculated on the anhydrous basis,in the Standard solution;Wis the weight,in mg,of Gadoteridol taken to prepare the Test solution;and rUand rSare the free gadolinium (III)peak responses obtained from the Test solutionand the Standard solution,respectively:not more than 0.01%is found.
Limit of regioisomer—
Using the chromatogram of the Assay preparationas obtained in the Assay,calculate the percentage of regioisomer peak at a relative retention time of about 1.2in relation to that of the gadoteridol peak by the formula:
100rR/(rR+rG),
in which rRand rGare the regioisomer and gadoteridol peak responses,respectively,in the Assay preparation:not more than 2.5%is found.
Chromatographic purity—
TEST1:GADOLINIUM-CONTAINING IMPURITIES—
Buffer solution—
Dissolve 10.2g of monobasic potassium phosphate,0.165g of dibasic potassium phosphate,and 1.76g of edetate disodium in 3000mLof water,and filter.
Mobile phase—
Prepare a degassed mixture of Buffer solutionand acetonitrile (98:2).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Diluent—
Transfer 3.40g of monobasic potassium phosphate,4.21g of dibasic potassium phosphate,and 0.584g of edetate disodium to a 1000-mLvolumetric flask,dissolve in and dilute with water to volume,and mix.
Standard stock solution 1—
Quantitatively dissolve an accurately weighed quantity of gadolinium (Gd III)acetate in water to obtain a solution having a concentration of about 0.4mg per mL.
Standard stock solution 2—
Prepare a solution of USP Gadoteridol Related Compound B RSin water to obtain a solution having a known concentration of about 0.6mg per mL.
Standard solution—
Transfer 1.0mLof Standard stock solution 1and 2.0mLof Standard stock solution 2to a 10-mLvolumetric flask,and dilute with Diluentto volume.Transfer 1.0mLof this solution to a vial,add 3.0mLof Diluent,and mix.
Test solution—
Transfer about 60mg of Gadoteridol,accurately weighed,to a vial,add 2.0mLof Diluent,and mix.Immediately place the vial in a bath maintained at about 5 .
.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a fluorometric detector operating at an excitation wavelength of 275nm and an emission wavelength of 314nm,and a 4.6-mm ×25-cm column that contains 5-µm packing L1.The flow rate is about 1mLper minute.Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the relative retention times are about 1.0for free gadolinium (III),1.6for 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid gadolinium sodium salt (gadoteridol related compound D),and 2.2for gadoteridol related compound B;and the relative standard deviation for replicate injections is not more than 5.0%.
Procedure—
Separately inject equal volumes (about 50µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure all of the peak responses.Allow the Test solutionto elute for not less than 1.3times the retention time of the gadoteridol peak.Calculate the percentage of gadoteridol related compound Din the portion of Gadoteridol taken by the formula:
200(C/W)(rU/rS),
in which Cis the concentration,in mg per mL,of USP Gadoteridol Related Compound B RS,calculated on the anhydrous basis,in the Standard solution;Wis the weight,in mg,of Gadoteridol taken to prepare the Test solution;and rUand rSare the gadoteridol related compound Dpeak responses obtained from the Test solutionand the Standard solution,respectively.Calculate the percentage of gadoteridol related compound Bin the portion of Gadoteridol taken by the formula:
200(C/W)(rU/rS),
in which Cis the concentration,in mg per mL,of USP Gadoteridol Related Compound B RS,calculated on the anhydrous basis,in the Standard solution;Wis the weight,in mg,of Gadoteridol taken to prepare the Test solution;and rUand rSare the gadoteridol related compound Bpeak responses obtained from the Test solutionand Standard solution,respectively.Calculate the percentage of any other impurity in the portion of Gadoteridol taken by the formula:
200(C/W)(ri/rS),
in which riis the peak response of any other impurity obtained from the Test solution;and rSis the peak response for gadoteridol related compound Bobtained from the Standard solution:not more than 0.1%of any individual impurity is found,and not more than 0.3%of gadolinium-containing impurities is found.
TEST2(NONGADOLINIUM-CONTAINING IMPURITIES)—
50mM Ammonium phosphate buffer—
Dissolve 17.25g of monobasic ammonium phosphate in 3000mLof water,and filter.
pH5.0Buffer—
Transfer 2000mLof 50mM Ammonium to a 2-Lbeaker,and adjust with ammonium hydroxide to a pHof 5.0.
pH7.0Buffer—
Transfer 1000mLof 50mM Ammonium to a 1-Lbeaker,and adjust with ammonium hydroxide to a pHof 7.0.
Mobile phase—
Prepare a filtered and degassed mixture of pH5.0bufferand acetonitrile (87:13).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard solution—
Dissolve an accurately weighed quantity of USP Gadoteridol Related Compound C RSin pH7.0Bufferto obtain a solution having a known concentration of about 5µg per mL.
System suitability solution—
Prepare a solution in pH7.0buffercontaining about 2mg of USP Gadoteridol RSand 0.002mg of USP Gadoteridol Related Compound C RSper mL.
Test solution—
Transfer about 50mg of Gadoteridol to a 25-mLvolumetric flask,dissolve in and dilute with pH7.0Bufferto volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm ×25-cm column that contains packing L18.The flow rate is about 1mLper minute.Chromatograph the System suitability solution,and record the responses as directed for Procedure:the relative retention times are about 1.0for gadoteridol,1.2for 1,4,7,10-tetraaza-1,4,7-tris-(carboxymethyl)-11-oxo-bicyclo [8.2.2]tetradecanium chloride (gadoteridol related compound F),1.5for 1,4,7,10-tetraaza-13-oxo-bicyclo [8.2.1]tridecane-4,7-diacetic acid (gadoteridol related compound E),and 1.7for gadoteridol related compound C;and the resolution,R,between gadoteridol and gadoteridol related compound Cis not less than 5.Chromatograph the Standard solutionas directed for Procedure:the relative standard deviation for replicate injections is not more than 4.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the peak responses.Allow the Test solutionto elute for not less than twice the retention time of the gadoteridol peak.Calculate the percentage of gadoteridol related compound Cin the portion of Gadoteridol taken by the formula:
2.5(C/W)(rU/rS),
in which Cis the concentration,in µg per mL,of USP Gadoteridol Related Compound C RSin the Standard solution;Wis the weight,in mg,of Gadoteridol taken to prepare the Test solution;and rUand rSare the peak responses for gadoteridol related compound Cobtained from the Test solutionand Standard solution,respectively.Calculate the percentages of gadoteridol related compound Fand gadoteridol related compound Ein the portion of Gadoteridol taken by the formula:
2.5(1/F)(C/W)(ri/rS),
in which Fis the relative response factor for gadoteridol related compound F(0.68)or for gadoteridol related compound E(1.7);riis the peak response for gadoteridol related compound Eor gadoteridol related compound Fin the Test solution;and C,W,and rSare as defined above.Calculate the percentage of any other impurity in the portion of Gadoteridol taken by the formula:
2.5(C/W)(ri/rS),
in which riis the response of any other impurity in the chromatogram of the Test solution;and C,W,and rSare as defined above:not more than 0.1%of any other impurity is found,and the total of nongadolinium-containing impurities is not more than 0.3%,calculated on the anhydrous basis.
Other requirements—
Where the label states that Gadoteridol is sterile,it meets the requirements for Sterility Tests á71ñand Bacterial endotoxinsunder Gadoteridol Injection.Where the label states that Gadoteridol must be subjected to further processing during the preparation of injectable dosage form,it meets the requirements for Bacterial endotoxinsunder Gadoteridol Injection.
Assay—
Buffer solution—
Dissolve 10.2g of monobasic potassium phosphate and 0.165g of dibasic potassium phosphate in 3Lof water,and filter.
Mobile phase—
Prepare a filtered and degassed mixture of Buffer solutionand acetonitrile (98:2).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Dissolve an accurately weighed quantity ofUSP Gadoteridol RSin Buffer solutionto obtain a solution having a known concentration of about 0.6mg per mL.
Assay preparation—
Transfer about 60mg of Gadoteridol,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with Buffer solutionto volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a fluorometric detector operating at an excitation wavelength of 275nm and an emission wavelength of 314nm,and a 4.6-mm ×25-cm column that contains 5-µm packing L1.The flow rate is about 1mLper minute.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the resolution,R,between the gadoteridol peak and the peak with a relative retention time of about 1.2is not less than 1.2;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 50µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of C17H29GdN4O7in the portion of Gadoteridol taken by the formula:
100C(rU/rS),
in which Cis the concentration of USP Gadoteridol RS,in mg per mL,in the Standard preparation;and rUand rSare the gadoteridol peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(RMI)Radiopharmaceuticals and Medical Imaging Agents
USP28–NF23Page 882
Pharmacopeial Forum:Volume No.29(6)Page 1890
Phone Number:1-301-816-8305