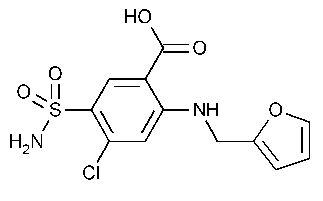
Furosemide
Benzoic acid,5-(aminosulfonyl)-4-chloro-2-[(2-furanylmethyl)amino]-.
4-Chloro-N-furfuryl-5-sulfamoylanthranilic acid [54-31-9].
»Furosemide contains not less than 98.0percent and not more than 101.0percent of C12H11ClN2O5S,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed,light-resistant containers.Store at 25 ,excursions permitted between 15
,excursions permitted between 15 and 30
and 30 .
.
USP Reference standards á11ñ—
USP Furosemide RS.USP Furosemide Related Compound A RS.USP Furosemide Related Compound B RS.
Identification—
A:Infrared Absorption á197Kñ.
B:Ultraviolet Absorption á197Uñ—
Solution:
8µg per mL.
Medium:
0.02Nsodium hydroxide.
Absorptivities at 271nm,calculated on the dried basis,do not differ by more than 3.0%.
C:
Dissolve about 5mg in 10mLof methanol.Transfer 1mLof this solution to a flask,add 10mLof 2.5Nhydrochloric acid,and reflux on a steam bath for 15minutes.Cool,and add 15mLof 1Nsodium hydroxide and 5mLof sodium nitrite solution (1in 1000).Allow the mixture to stand for 3minutes,add 5mLof ammonium sulfamate solution (1in 200),mix,and add 5mLof freshly prepared N-(1-naphthyl)ethylenediamine dihydrochloride solution (1in 1000):a red to red-violet color is produced.
Loss on drying á731ñ—
Dry it at 105 for 3hours:it loses not more than 1.0%of its weight.
for 3hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.002%.
Related compounds—
[NOTE—Protect Furosemide solutions from exposure to light.]
Mobile phase—
Prepare a filtered and degassed mixture of water,tetrahydrofuran,and glacial acetic acid (70:30:1).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Diluting solution—
Dilute 22mLof glacial acetic acid with a mixture of acetonitrile and water (50:50)to 1000mL,and mix.
System suitability solution—
Dissolve suitable quantities of USP Furosemide RSand USP Furosemide Related Compound A RSin Diluting solutionto obtain a solution containing about 20µg per mLand 12µg per mL,respectively.
Standard solution—
Prepare a solution in Diluting solutioncontaining 5.0µg each of USP Furosemide Related Compound A RSand USP Furosemide Related Compound B RSper mL.
Test solution—
Transfer an accurately weighed quantity of Furosemide to a suitable volumetric flask,dissolve in and dilute with Diluting solutionto volume to obtain a solution having a concentration of about 1.0mg per mL,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a detector capable of recording at both 254nm and 272nm and a 4.6-mm ×25-cm column that contains packing L1.[NOTE—The 2,4-dichloro-5-sulfamoylbenzoic acid impurity does not respond at 272nm and the 2,4-bis(furfurylamino)-5-sulfamoylbenzoic acid impurity has a very intense absorbance at 254nm.]The flow rate is about 1.0mLper minute.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the resolution,R,between furosemide and furosemide related compound Ais not less than 2.5;and the relative standard deviation determined from furosemide is not more than 2.0%.[NOTE—The response for furosemide is at 254nm.]
Procedure—
Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the areas for the major peaks.[NOTE—The chromatographic run time is not less than 2.5times the retention time of the furosemide peak.]The sum of the responses at 254nm of those peaks eluting before furosemide in the chromatogram obtained from the Test solutionis not more than the response at 254nm of the furosemide related compound Bpeak in the chromatogram obtained from the Standard solution(0.5%).The sum of the responses at 272nm of those peaks eluting after furosemide in the chromatogram obtained from the Test solutionis not more than the response at 272nm of the furosemide related compound Apeak in the chromatogram obtained from the Standard solution(0.5%).
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Assay—
Dissolve about 600mg of Furosemide,accurately weighed,in 50mLof dimethylformamide to which has been added 3drops of bromothymol blue TS,and which previously has been neutralized with 0.1Nsodium hydroxide.Titrate with 0.1Nsodium hydroxide VSto a blue endpoint.Each mLof 0.1Nsodium hydroxide is equivalent to 33.07mg of C12H11ClN2O5S.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 876
Pharmacopeial Forum:Volume No.29(5)Page 1497
Phone Number:1-301-816-8305