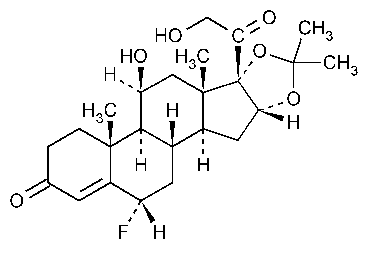
Flurandrenolide
Pregn-4-ene-3,20-dione,6-fluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-,6a11b,16a)-.
6a-Fluoro-11b,16a,17,21-tetrahydroxypregn-4-ene-3,20-dione,cyclic 16,17-acetal with acetone [1524-88-5].
»Flurandrenolide contains not less than 97.0percent and not more than 102.0percent of C24H33FO6,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers in a cold place,protected from light.
Identification—
A:
Infrared Absorption á197Kñ.
B:
Ultraviolet Absorption á197Uñ—
Solution:
20µg per mL.
Medium:
methanol.
Absorptivities at 237nm,calculated on the dried basis,do not differ by more than 3.0%.
Specific rotation á781Sñ:
between +145 and +153
and +153 .
.
Test solution:
10mg per mL,in chloroform.
Loss on drying á731ñ—
Dry it in vacuum at 105 for 4hours:it loses not more than 1.0%of its weight.
for 4hours:it loses not more than 1.0%of its weight.
Ordinary impurities á466ñ—
Test solution:
methanol.
Standard solution:
methanol.
Application volume:
10µL.
Eluant:
a mixture of toluene and isopropyl alcohol (90:10),in a nonequilibrated chamber.
Visualization:
1.
Assay—
Mobile phase—
Prepare a filtered and degassed mixture of methanol and water (60:40).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Internal standard solution—
Dissolve Prednisone in Mobile phase,with the aid of sonication,to obtain a solution containing about 1mg per mL.
Standard preparation—
Transfer about 5mg of USP Flurandrenolide RS,accurately weighed,to a 10-mLvolumetric flask,add 2.0mLof Internal standard solution,dilute with Mobile phaseto volume,sonicate to aid solution,and mix to obtain a solution having a known concentration of about 0.5mg of USP Flurandrenolide RSper mL.
Assay preparation—
Transfer about 5mg of Flurandrenolide,accurately weighed,to a 10-mLvolumetric flask,add 2.0mLof Internal standard solution,dilute with Mobile phaseto volume,sonicate to aid solution,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 240-nm detector and a 4-mm ×25-cm column that contains packing L1.The flow rate is about 1mLper minute.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the order of elution is prednisone followed by flurandrenolide,the resolution;R,between the analyte and internal standard is not less than 2.0;and the relative standard deviation for replicate injections is not more than 3.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.The relative retention times are about 0.5for prednisone and 1.0for flurandrenolide.Calculate the quantity,in mg,of C24H33FO6in the portion of Flurandrenolide taken by the formula:
10C(RU/RS),
in which Cis the concentration,in mg per mL,of USP Flurandrenolide RSin the Standard preparation;and RUand RSare the peak response ratios obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 862
Phone Number:1-301-816-8139