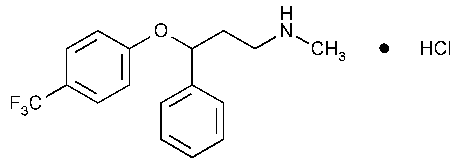
Fluoxetine Hydrochloride
Benzenepropanamine,N-methyl-g-[4-(trifluoromethyl)phenoxy]-,hydrochloride,(±).
(±)-N-Methyl-3-phenyl-3-[(a,a,a-trifluoro-p-tolyl)oxy]propylamine,hydrochloride [59333-67-4].
»Fluoxetine Hydrochloride contains not less than 98.0percent and not more than 102.0percent of C17H18F3NO·HCl,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
USP Reference standards á11ñ—
USP Fluoxetine Hydrochloride RS.USP Fluoxetine Related Compound A RS.USP Fluoxetine Related Compound B RS.
Identification—
A:
Infrared Absorption á197Kñ.
B:
Asolution meets the requirements of the tests for Chloride á191ñ.
Water,Method Iá921ñ:
not more than 0.5%.
Heavy metals,Method IIá231ñ:
0.003%.
Related compounds—
Mobile phase—
Proceed as directed in the Assay.
Test solution 1—
Transfer about 56mg of Fluoxetine Hydrochloride,accurately weighed,to a 10-mLvolumetric flask,dissolve in and dilute with Mobile phaseto volume,and mix.
Test solution 2—
Transfer 2mLof Test solution 1,accurately measured,to a 10-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
System suitability solution—
Dissolve about 22mg of USP Fluoxetine Hydrochloride RSin 10mLof 1Nsulfuric acid,and heat to 85 for 3hours.Cool,transfer 0.4mLof this solution to a 25-mLvolumetric flask,and add about 28mg of USP Fluoxetine Hydrochloride RS,1mg of USP Fluoxetine Related Compound A RS,and 1mg of USP Fluoxetine Related Compound B RS.Dilute with Mobile phaseto volume,and mix.
for 3hours.Cool,transfer 0.4mLof this solution to a 25-mLvolumetric flask,and add about 28mg of USP Fluoxetine Hydrochloride RS,1mg of USP Fluoxetine Related Compound A RS,and 1mg of USP Fluoxetine Related Compound B RS.Dilute with Mobile phaseto volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 215-nm detector and a 4.6-mm ×25-cm column that contains 5-µm base-deactivated packing L7.The flow rate is about 1mLper minute.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.24for a-[2-(methylamino)ethyl]benzenemethanol (if present),0.27for fluoxetine related compound B(if present),0.94for fluoxetine related compound A,1.0for fluoxetine,and 2.17for 4-trifluoromethylphenol;and the ratio of the height of the fluoxetine related compound Apeak to the depth of the valley between the fluoxetine and fluoxetine related compound Apeaks (measured from the fluoxetine related compound Apeak height)is not more than 1.1.
Procedure—
Separately inject equal volumes (about 10µL)of Test solution 1and Test solution 2into the chromatograph,record the chromatograms for not less than twice the elution time for fluoxetine,and measure the peak responses.Calculate the percentage of fluoxetine related compound Ain the portion of Fluoxetine Hydrochloride taken by the formula:
100rA/(rA+rU),
in which rAis the peak response of fluoxetine related compound Aobtained from Test solution 2;and rUis the peak response of fluoxetine obtained from Test solution 2.
Calculate the percentage of each of the other impurities in the portion of Fluoxetine Hydrochloride taken by the formula:
100ri/(rs+5rU),
in which riis the peak response for each impurity obtained from Test solution 1;rsis the sum of the responses of all the peaks,excluding fluoxetine,obtained from Test solution 1;and rUis as defined above:not more than 0.15%of fluoxetine related compound Ais found;not more than 0.25%of a-[2-(methylamino)ethyl]benzenemethanol is found;not more than 0.25%of fluoxetine related compound Bis found;and not more than 0.1%of any other individual impurity is found.The sum of all impurities found is not more than 0.5%.
Organic volatile impurities,Method IVá467ñ:
meets the requirements.
Assay—
Triethylamine buffer—
Transfer about 10mLof triethylamine,accurately measured,to a suitable container,add about 980mLof water,and adjust with phosphoric acid to a pHof 6.0.
Mobile phase—
Prepare a filtered and degassed mixture of Triethylamine buffer,stabilizer-free tetrahydrofuran,and methanol (6:3:1).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Dissolve an accurately weighed quantity of USP Fluoxetine Hydrochloride RSin Mobile phase,and dilute quantitatively,and stepwise if necessary,with Mobile phaseto obtain a solution having a known concentration of about 0.11mg per mL.
Assay preparation—
Transfer about 11mg of Fluoxetine Hydrochloride,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with Mobile phaseto volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 227-nm detector and a 4.6-mm ×25-cm column that contains 5-µm base-deactivated packing L7.The flow rate is about 1mLper minute.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the tailing factor is not more than 2.0,and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of C17H18F3NO·HCl in the portion of Fluoxetine Hydrochloride taken by the formula:
100C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Fluoxetine Hydrochloride RSin the Standard preparation;and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Salvador C.Salado,M.S.,Scientist and Latin American Liaison
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 853
Pharmacopeial Forum:Volume No.30(3)Page 848
Phone Number:1-301-816-8165