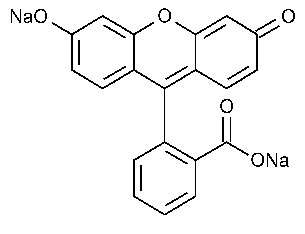
Fluorescein Sodium
Spiro[isobenzofuran-1(3H),9¢-[9H]xanthene]-3-one,3¢6¢-dihydroxy,disodium salt.
Fluorescein disodium salt [518-47-8].
»Fluorescein Sodium contains not less than 90.0percent and not more than 102.0percent of C20H10Na2O5,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
Asolution of it is strongly fluorescent,even in extreme dilution.The fluorescence disappears when the solution is made acid,and reappears when the solution is again made alkaline.
B:
The residue remaining after the incineration of it responds to the tests for Sodium á191ñ.
C:
Place 1drop of a solution (1in 2000)upon a piece of filter paper:a yellow spot is produced and,when exposed while moist to the vapor of bromine for 1minute and then to ammonia vapor,it becomes deep pink in color.
Water,Method Iá921ñ:
not more than 17.0%.
Zinc—
Dissolve 100mg in 10mLof a saturated solution of sodium chloride,add 2mLof 3Nhydrochloric acid,shake well,filter,and add 1mLof potassium ferrocyanide TSto the filtrate:no turbidity is produced.
Acriflavine—
Dissolve 10mg in 5mLof water,and add a few drops of sodium salicylate solution (1in 10):no precipitate is formed.
Assay—
Standard preparation—
Dissolve an accurately weighed quantity of USP Diacetylfluorescein RSin 10mLof alcohol contained in a 100-mLvolumetric flask.[NOTE—110.7mg of anhydrous USP Diacetylfluorescein RSis equivalent to 100.0mg of fluorescein sodium.]Add 2mLof 2.5Nsodium hydroxide,and heat on a steam bath at about the boiling temperature for 20minutes,with frequent swirling.Cool,dilute with water to volume,and mix.Transfer a suitable aliquot to a volumetric flask,and dilute with water to volume to obtain a solution having a concentration of 1µg of fluorescein sodium per mL.Transfer a 3-mLaliquot to a 100-mLvolumetric flask containing 20mLof pH9.0alkaline borate buffer (see Buffer Solutionsin the section Reagents,Indicators,and Solutions),dilute with water to volume,and mix.The concentration of fluorescein sodium in the Standard preparationis 0.03µg per mL.
Assay preparation—
Dissolve about 100mg of Fluorescein Sodium,accurately weighed,in water,and dilute quantitatively and stepwise with water to obtain a solution having a concentration of 1µg per mL.Transfer 3.0mLof this solution to a 100-mLvolumetric flask containing 20mLof pH9.0alkaline borate buffer (see Buffer Solutionsin the section Reagents,Indicators,and Solutions),dilute with water to volume,and mix.
Procedure—
Concomitantly determine the fluorescence intensities,I,of the Standard preparationand the Assay preparationin a fluorometer at an excitation wavelength of 485nm and an emission wavelength at 515nm.Calculate the quantity,in mg,of C20H10Na2O5in the Fluorescein Sodium taken by the formula:
3333C(IU/IS),
in which Cis the concentration,in µg per mL,of fluorescein sodium in the Standard preparation,and IUand ISare the fluorescence values observed for the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(PA6)Pharmaceutical Analysis 6
USP28–NF23Page 844
Phone Number:1-301-816-8389