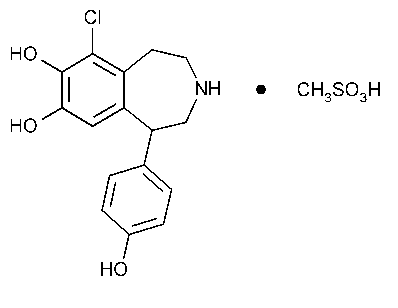
Fenoldopam Mesylate
C16H16ClNO3·CH4SO3 401.87
1H-3-Benzazepine-7,8-diol,6-chloro-2,3,4,5-tetrahydro-1-(4-hydroxyphenyl)-,methanesulfonate (salt).
6-Chloro-2,3,4,5-tetrahydro-1-(p-hydroxyphenyl)-1H-3-benzazepine-7,8-diol methanesulfonate (salt) [67227-57-0].
»Fenoldopam Mesylate contains not less than 98.0percent and not more than 102.0percent of C16H16ClNO3·CH4SO3,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers,protected from moisture.Store at 25 ,excursions permitted between 15
,excursions permitted between 15 and 30
and 30 .
.
Identification—
A:Infrared Absorption á197Kñ.
B:
The retention time of the major peak in the chromatogram of theAssay preparationcorresponds to that in the chromatogram of theStandard preparation,as obtained in theAssay.
Water,Method Iá921ñ:
not more than 1.0%.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.002%.
Limit of iodide—
Mobile phase—
Prepare a filtered and degassed solution containing about 0.94g of sodium bicarbonate,0.952g of sodium carbonate,0.38g of 4-cyanophenol,and 80mLof acetonitrile in 4Lof water.Make adjustments if necessary (seeSystem SuitabilityunderChromatography á621ñ).
Standard stock solution—
Transfer about 118.1mg of sodium iodide,accurately weighed,to a 1000-mLvolumetric flask.Dissolve in and dilute with water to volume,and mix to obtain a solution containing the equivalent of 100µg of iodide per mL.
Standard solutions—
Pipet 2.0mL,4.0mL,6.0mL,and 8.0mLof the Standard stock solutioninto separate 100-mLvolumetric flasks,dilute with water to volume,and mix to obtain solutions having known concentrations of about 2µg,4µg,6µg,and 8µg of iodide per mL.
Test solution—
Transfer about 300mg of Fenoldopam Mesylate,accurately weighed,to a 100-mLvolumetric flask.Dissolve in and dilute with water to volume,and mix.
Chromatographic system—
The ion chromatograph is equipped with a conductivity detector,a 4-mm ×3.5-cm anion-exchange guard column,a 4-mm ×15-cm anion-exchange analytical column,and a micromembrane anion suppressor column.The flow rate is about 2.0mLper minute.The regeneration solution for the suppressor column is a 0.050Msulfuric acid solution,flowing at a rate of 5mLper minute.Chromatograph the 6µg per mLStandard solution,and record the peak responses as directed forProcedure:the relative standard deviation for replicate injections is not more than 5.0%.
Procedure—
Separately inject equal volumes (about 100µL)of each of theStandard solutionsand theTest solutioninto the chromatograph,record the chromatograms,and measure the heights of peak responses.Plot the response of each of theStandard solutionsversus the concentration,and draw the straight line best fitting the plotted points.From the graph so obtained,determine the quantity of iodide in the portion of Fenoldopam Mesylate taken:not more than 0.2%is found.
Related compounds—
Buffer solution,System suitability stock solution,and System suitability solution—
Proceed as directed in theAssay.
Solution A—
UseMobile phaseas prepared in theAssay.
Solution B—
Use filtered and degassed methanol.
Mobile phase—
Use variable mixtures ofSolution AandSolution Bas directed forChromatographic system.Make adjustments if necessary (seeSystem SuitabilityunderChromatography á621ñ).
Test solution—
Use theSystem suitability stock solution.
Chromatographic system—
Proceed as directed in theAssay,except to program the chromatograph as follows.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0 | 100 | 0 | equilibration |
| 0–30 | 100 | 0 | isocratic |
| 30–60 | 100®70 | 0®30 | linear gradient |
Procedure—
Inject a volume (about 20µL)of theTest solutioninto the chromatograph,record the chromatogram,and measure the peak responses.Calculate the percentage of each impurity in the portion of Fenoldopam Mesylate taken by the formula:
100(ri/rs),
in which riis the peak response for each impurity;and rsis the sum of the responses of all the peaks:not more than 0.3%of fenoldopam related compound Ais found;not more than 0.1%of any other individual impurity is found;and not more than 1.0%of total impurities is found.
Limit of residual solvents—
Internal standard solution—
Prepare a solution,in organic-free water,containing 10mg of n-butanol per mL.Transfer 100µLof this solution to a 10-mLvolumetric flask,dilute with dimethylsulfoxide to volume,and mix.
Standard solution—
Prepare a solution,in organic-free water,containing 10mg each of n-propanol,isopropyl alcohol,and dimethylformamide per mL.Transfer 100µLof this solution to a 10-mLvolumetric flask,dilute withInternal standard solutionto volume,and mix.
Test solution—
Transfer about 50mg of Fenoldopam Mesylate,accurately weighed,to a 1-mLvolumetric flask.Dilute withInternal standard solutionto volume,and sonicate to dissolve completely.
Chromatographic system(see Chromatography á621ñ)—
The gas chromatograph is equipped with a flame-ionization detector,a 0.32-mm ×30-m fused-silica capillary column coated with a 1.8-µm film of stationary phase G43,and a split injection system.The carrier gas is helium,flowing at a rate of about 1mLper minute through the column and a split ratio of about 50:1.The injection port and the detector temperatures are maintained at 140 and 260
and 260 ,respectively.The column temperature is programmed as follows.It is maintained for 12minutes at 40
,respectively.The column temperature is programmed as follows.It is maintained for 12minutes at 40 ,then increased at a rate of 8
,then increased at a rate of 8 per minute to 120
per minute to 120 ,held for 0.1minute,then increased at a rate of 25
,held for 0.1minute,then increased at a rate of 25 per minute to 180
per minute to 180 ,and maintained at that temperature for 8minutes.
,and maintained at that temperature for 8minutes.
Procedure—
Separately inject equal volumes (about 1µL)of theStandard solution,dimethylsulfoxide,and theTest solutioninto the chromatograph,record the chromatograms,and measure the peak areas.Identify,based on retention time,any peaks present in the chromatogram of theTest solution.Calculate the response factor,F,for each solvent in theStandard solutionby the formula:
(WR/WI)(rI/rR),
in which WRis the weight,in mg,of the solvent of interest;WIis the weight,in mg,of the internal standard taken to prepare the Internal standard solution;and rIand rRare the peak responses for the internal standard and the solvent of interest,respectively,obtained from theStandard solution.Calculate the percentage,by weight,of each solvent found in theTest solutionby the formula:
100FD(rI/rS)(WI/WD),
in whichFis the average response factor for the solvent of interest obtained from all injections of the Standard solution;Dis the dilution factor for the internal standard in theTest solution(i.e.,0.0001);rIand rSare the peak responses for the solvent of interest and the internal standard,respectively,obtained from theTest solution;WIis the weight,in mg,of the internal standard taken to prepare theInternal standard solution;andWDis the weight,in mg,of Fenoldopam Mesylate taken to prepare theTest solution:not more than 0.2%of total residual solvents is found.
Assay—
Buffer solution—
Transfer about 16.33g of monobasic potassium phosphate and 2mLof triethylamine to a 2-Lvolumetric flask,and dissolve in 1800mLof water.Adjust with phosphoric acid to a pHof 2.5,dilute with water to volume,and mix.
Mobile phase—
Prepare a filtered and degassed mixture ofBuffer solutionand methanol (19:1).Make adjustments if necessary (seeSystem SuitabilityunderChromatography á621ñ).
System suitability stock solution—
Transfer about 50mg of Fenoldopam Mesylate,accurately weighed,to a 50-mLvolumetric flask.With the aid of an ultrasonic bath,dissolve in and dilute withMobile phaseto volume,and mix.
System suitability solution—
Transfer about 5mg of USP Fenoldopam Related Compound A RS,accurately weighed,to a 50-mLvolumetric flask.Add about 25mLofMobile phase,and sonicate to dissolve.Add 5mLof theSystem suitability stock solution,dilute withMobile phaseto volume,and mix.
Standard preparation—
Dissolve an accurately weighed quantity of USP Fenoldopam Mesylate RSinMobile phaseto obtain a solution having a known concentration of about 0.1mg per mL.
Assay preparation—
Transfer 5.0mLof theSystem suitability stock solution,accurately measured,to a 50-mLvolumetric flask,dilute withMobile phaseto volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 225-nm detector and a 3.9-mm ×30-cm column that contains packing L11.The flow rate is about 1.7mLper minute.Chromatograph theSystem suitability solution,and record the peak responses as directed forProcedure:the resolution,R,between fenoldopam and fenoldopam related compound Ais not less than 1.5;the column efficiency is not less than 2000theoretical plates;the tailing factor is not more than 1.3;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20µL)of theStandard preparationand theAssay preparationinto the chromatograph,record the chromatograms,and measure the peak responses.Calculate the quantity,in mg,of C16H16ClNO3·CH4SO3in the portion of Fenoldopam Mesylate taken by the formula:
500C(rU/rS),
in whichCis the concentration,in mg per mL,of USP Fenoldopam Mesylate RSin theStandard preparation;andrUandrSare the peak responses for fenoldopam obtained from theAssay preparationand theStandard preparation,respectively.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 810
Pharmacopeial Forum:Volume No.29(5)Page 1479
Phone Number:1-301-816-8305