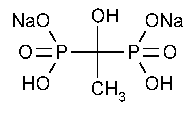
Etidronate Disodium
Phosphonic acid,(1-hydroxyethylidene)bis-,disodium salt.
Disodium dihydrogen (1-hydroxyethylidene)diphosphonate [7414-83-7].
»Etidronate Disodium contains not less than 97.0percent and not more than 101.0percent of C2H6Na2O7P2,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
Infrared Absorption á197ñ—The spectra of trifluorovinyl chloride polymer and mineral oil dispersions of it,separately prepared from a test specimen recrystallized from water and dried at 105 for 1hour,exhibit maxima in the regions of 4000to 1350cm-1and 1350to 450cm-1,respectively,only at the same wavelengths as those of similar preparations of USP Etidronate Disodium RS.
for 1hour,exhibit maxima in the regions of 4000to 1350cm-1and 1350to 450cm-1,respectively,only at the same wavelengths as those of similar preparations of USP Etidronate Disodium RS.
B:
Asolution (1in 100)responds to the flame test for Sodium á191ñ.
pHá791ñ:
between 4.2and 5.2,in a solution (1in 100).
Water,Method Ic á921ñ:
not more than 5.0%,determined on a preparation containing 100mg of finely powdered etidronate disodium in 10mLof a mixture of acetic acid and formamide (1:1).
Phosphite—
Buffer solution—
Dissolve 6.9g of monobasic sodium phosphate in 500mLof water,add 400mLof 0.1Nsodium hydroxide,and mix.
Test preparation—
Transfer about 3.5g of Etidronate Disodium,accurately weighed,to a glass-stoppered,250-mLconical flask,and dissolve in 70mLof water.
Procedure—
Add 20mLof Buffer solutionto the Test preparation,and adjust with sodium hydroxide solution (25in 100),using a pHmeter,to a pHof 7.3±0.2.Add 20mLof iodine TSin divided portions,with mixing,and insert the stopper securely in the flask.[NOTE—If more than half of the iodine TSis absorbed (decolorized)by the portion of Etidronate Disodium taken,discard the mixture,and use a Test preparation containing a smaller quantity of Etidronate Disodium.]Allow to stand for 3hours protected from light,and adjust with 6Nacetic acid to a pHof 4.5±0.2.Titrate with 0.1Nsodium thiosulfate VS.When the iodine color becomes pale,add 2mLof starch TS,and continue the titration until the blue color is discharged.Perform a blank determination (see Titrimetry á541ñ),and make any necessary correction.Calculate the percentage of phosphite in the portion of Etidronate Disodium taken by the formula:
0.520V/W,
in which Vis the volume,in mL,of titrant consumed,and Wis the weight,in g,of Etidronate Disodium taken:not more than 1.0%is found.
Heavy metals,Method IIá231ñ—
Use 0.5g of Etidronate Disodium for the Test Preparationand 2.5mLof Standard Lead Solutionfor the Standard Preparation.Transfer the Test Preparationand the Standard Preparationto separate quartz crucibles,add 0.5g of magnesium oxide to each crucible,and mix.Evaporate the Standard Preparationto dryness at 110 for 1hour,and ignite each crucible over a flame to a light gray color.Ignite at 800
for 1hour,and ignite each crucible over a flame to a light gray color.Ignite at 800 for 1hour,cool,and dissolve the residues by the dropwise addition of hydrochloric acid,and add 3mLof water.Adjust with ammonia TSto a pHof 8.5,then adjust with acetic acid to a pHof 4.Make a final pHadjustment to 3.4±0.05,using dilute hydrochloric acid.Filter into 50-mLcolor comparison tubes,and dilute with water to 40mL.The limit is 0.005%.
for 1hour,cool,and dissolve the residues by the dropwise addition of hydrochloric acid,and add 3mLof water.Adjust with ammonia TSto a pHof 8.5,then adjust with acetic acid to a pHof 4.Make a final pHadjustment to 3.4±0.05,using dilute hydrochloric acid.Filter into 50-mLcolor comparison tubes,and dilute with water to 40mL.The limit is 0.005%.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Titrant—
Transfer 6.9g of thorium nitrate,accurately weighed,to a 1000-mLbeaker,and dissolve in 25mLof 1Nnitric acid.Dissolve 4.7g of (1,2-cyclohexylenedinitrilo)tetraacetic acid in 41mLof 1Nsodium hydroxide,and transfer to the 1000-mLbeaker.Add about 600mLof water,and adjust with methenamine to a pHof between 5.0and 5.5.Transfer to a 1000-mLvolumetric flask,dilute with water to volume,and mix.Allow to stand for 1week before use.This solution is about 0.0125Min thorium-cyclohexylenedinitrilotetraacetic acid complex.Each mLis equivalent to approximately 1.5mg of C2H6Na2O7P2.
Standard preparation—
Transfer 180mg of USP Etidronic Acid Monohydrate RSto a 100-mLvolumetric flask,dissolve in 50mLof water,add 16mLof 0.1Nsodium hydroxide,dilute with water to volume,and mix.
Assay preparation—
Transfer about 400mg of Etidronate Disodium,accurately weighed,to a 200-mLvolumetric flask,dissolve in 100mLof water,dilute with water to volume,and mix.
Procedure—
Pipet 15mLeach of the Standard preparationand the Assay preparationinto separate 100-mLbeakers.To each beaker add 20mLof water,1mLof a 0.1%solution of xylenol orange,and 2mLof methenamine solution (20in 100),and mix.Adjust with 0.1Nnitric acid to a pHof 6.50±0.05.Concomitantly titrate the resulting solutions to a reddish violet endpoint.Calculate the quantity,in mg,of C2H6Na2O7P2in the portion of Etidronate Disodium taken by the formula:
2(249.99/224.05)WS(VU/VS),
in which 249.99and 224.05are the molecular weights of etidronate disodium and etidronic acid monohydrate,respectively;WSis the weight,in mg,of USP Etidronic Acid Monohydrate RStaken,and VUand VSare the volumes,in mL,of Titrantconsumed by the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Elena Gonikberg,Ph.D.,Scientist
Expert Committee:(PA4)Pharmaceutical Analysis 4
USP28–NF23Page 798
Pharmacopeial Forum:Volume No.30(5)Page 1616
Phone Number:1-301-816-8251