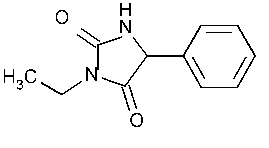
Ethotoin
2,4-Imidazolidinedione,3-ethyl-5-phenyl-,(±)-.
(±)-3-Ethyl-5-phenylhydantoin [86-35-1].
»Ethotoin contains not less than 97.5percent and not more than 102.0percent of C11H12N2O2,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification—
B:
The retention time of the major peak in the chromatogram of the Assay preparationcorresponds to that in the chromatogram of the Standard preparation,as obtained in the Assay.
Loss on drying á731ñ—
Dry it in vacuum at 60 for 4hours:it loses not more than 1.0%of its weight.
for 4hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Chloride—
Transfer 1.0g of Ethotoin to a suitable separator,and dissolve in 50mLof ether.Extract with three 15-mLportions of water,collect the combined extracts in a beaker,heat on a steam bath to expel any traces of ether,and allow to cool to room temperature.Transfer the solution to a 50-mLcolor-comparison tube,add 2Nnitric acid until the solution is acidic,add 1mLof 2Nnitric acid in excess,mix,add 1mLof silver nitrate TS,dilute with water to 50mL,and allow to stand for 5minutes,protected from direct sunlight.The turbidity produced does not exceed that of a solution prepared by mixing 2mLof freshly prepared 0.002Nhydrochloric acid,1mLof 2Nnitric acid,1mLof silver nitrate TS,and 46mLof water (0.014%).
Heavy metals,Method IIá231ñ:
0.002%.
Related compounds—
Buffer solution—
Dissolve about 1g of monobasic sodium phosphate in 1liter of water.Adjust the solution with 1.5Mphosphoric acid to a pHof 3.5±0.1.
Diluent—
Prepare a mixture of Buffer solutionand methanol (65:35).
Solution A—
Prepare a filtered and degassed mixture of Buffer solutionand acetonitrile (80:20).
Solution B—
Prepare a filtered and degassed mixture of Buffer solutionand acetonitrile (60:40).
Mobile phase—
Use variable mixtures of Solution Aand Solution Bas directed for Chromatographic system.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard solution—
Dissolve an accurately weighed quantity of USP Ethotoin RS,and dilute quantitatively,and stepwise if necessary,with Diluentto obtain a solution having a known concentration of about 2.5µg of ethotoin per mL.
Test solution—
Transfer about 50mg of Ethotoin,accurately weighed,to a 200-mLvolumetric flask,dissolve in about 100mLof Diluentwith sonication,dilute with Diluentto volume,and mix.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 210-nm detector and a 4.6-mm ×15-cm column that contains 5-µm packing L1.The column temperature is maintained at 40 .The flow rate is about 0.8mLper minute.The chromatograph is programmed as follows.
.The flow rate is about 0.8mLper minute.The chromatograph is programmed as follows.
Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the tailing factor is not more than 2.0for ethotoin;and the relative standard deviation for replicate injections is not more than 1.0%.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0 | 100 | 0 | equilibration |
| 0–10 | 100 | 0 | isocratic |
| 10–30 | 100®0 | 0®100 | linear gradient |
| 30–40 | 0 | 100 | isocratic |
| 40–42 | 0®100 | 100®0 | linear gradient |
| 42–55 | 100 | 0 | re-equilibration |
Procedure—
Separately inject equal volumes (about 100µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the responses for the major peaks [NOTE—Discard any peak due to the Diluent].Calculate the percentage of any impurity in the portion of Ethotoin taken by the formula:
(20,000/F)(C/W)(ri/rS),
in which Cis the concentration,in mg per mL,of USP Ethotoin RSin the Standard solution;Fis the response factor for the impurity as shown in the table below;Wis the weight,in mg,on the anhydrous basis,of the portion of Ethotoin taken;riis the peak area for any impurity in the Test solution;and rSis the peak area for ethotoin in the Standard solution.The impurities meet the requirements given in the table below.
| Compound name | RRT1 | RRF2 | Limit (%) |
| 5-Phenylhydantoin | about 0.4 | 1.0 | 1.5 |
| 3-Methyl-5-phenylhydantoin | about 0.6 | 1.0 | 0.9 |
| Ethotoin | 1.0 | — | — |
| Ethotoin/5-Phenylhydantoin dimer | about 1.9 | 1.0 | 0.3 |
| Ethotoin dimer | about 2.5 | 0.58 | 0.4 |
| Unknown impurities | — | 1.0 | 0.1Individual |
| 1.0Total Unknown | |||
| Total | — | — | 2.0 |
|
1
RRT—Relative retention time
|
|||
|
2
RRF—Relative response factor
|
|||
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Assay—
Mobile phase—
Dissolve 0.65g of monobasic potassium phosphate in 600mLof water,adjust with phosphoric acid solution (1in 10)to a pHof 3.5±0.1,and dilute with water to 650mL.Add 350mLof methanol,mix,filter through a membrane filter of 0.5µm or finer porosity,and degas.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
5-Phenylhydantoin stock solution—
Dissolve,with the aid of sonication if necessary,an accurately weighed quantity of USP5-Phenylhydantoin RSin Mobile phaseto obtain a solution having a known concentration of about 0.37mg per mL.
Standard preparation—
Dissolve,with the aid of sonication if necessary,an accurately weighed quantity of USP Ethotoin RSin Mobile phaseto obtain a solution having a known concentration of about 0.25mg of ethotoin per mL.
System suitability solution—
Transfer 25mg of USP Ethotoin RS,accurately weighed,to a 100-mLvolumetric flask,add about 1.0mLof 5-Phenylhydantoin stock solution,add Mobile phaseto volume,and sonicate to dissolve.
Assay preparation—
Transfer about 50mg of Ethotoin,accurately weighed,to a 200-mLvolumetric flask,dissolve in Mobile phase,dilute with Mobile phaseto volume,and sonicate to dissolve.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 210-nm detector and a 4.6-mm ×30-cm column that contains packing L1.The flow rate is about 1.5mLper minute.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the resolution,R,between the 5-phenylhydantoin and ethotoin peaks is not less than 6.0.The relative retention times are about 0.4for 5-phenylhydantoin and 1.0for ethotoin.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 3.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of C11H12N2O2in the portion of Ethotoin taken by the formula:
200C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Ethotoin RSin the Standard preparation;and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Salvador C.Salado,M.S.,Scientist and Latin American Liaison
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 794
Pharmacopeial Forum:Volume No.29(1)Page 66
Phone Number:1-301-816-8165