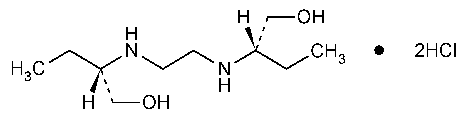
Ethambutol Hydrochloride
1-Butanol,2,2¢-(1,2-ethanediyldiimino)bis-,dihydrochloride,[S-(R*,R*)]-.
(+)-2,2¢-(Ethylenediimino)-di-1-butanol dihydrochloride [1070-11-7].
»Ethambutol Hydrochloride contains not less than 98.0percent and not more than 100.5percent of C10H24N2O2·2HCl,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
Infrared Absorption á197Kñ.
B:
Asolution (1in 10)responds to the tests for Chloride á191ñ.
Specific rotation á781Sñ:
between +6.0 and +6.7
and +6.7 .
.
Test solution:
100mg per mL,in water.
Loss on drying á731ñ—
Dry it at 105 for 2hours:it loses not more than 0.5%of its weight.
for 2hours:it loses not more than 0.5%of its weight.
Heavy metals,Method IIá231ñ:
0.002%.
Limit of aminobutanol—
Borate buffer—
Transfer about 1.24g of boric acid to a 100-mLvolumetric flask,dissolve in 90mLof water,and adjust with 5Nsodium hydroxide to a pHof 9.0,dilute with water to volume,and mix.
Standard solution—
Dissolve an accurately weighed quantity of USP Aminobutanol RSin water,and dilute quantitatively,and stepwise if necessary,to obtain a solution having a known concentration of about 5µg per mL.
Fluorescamine solution—
Dissolve 5mg of fluorescamine in 50mLof acetone in a glass-stoppered,graduated cylinder.
Test solution—
Transfer about 50mg of Ethambutol Hydrochloride,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with water to volume,and mix.
Procedure—
Pipet a 10-mLportion of Test solutioninto a glass-stoppered,100-mLconical flask,and add 10mLof water and 20mLof Borate buffer.To another 100-mLflask add 10.0mLof Test solution,10.0mLof Standard solution,and 20mLof Borate buffer.Place the flasks on a magnetic stirrer,and while the contents are being stirred rapidly,add 10mLof Fluorescamine solutionrapidly.Insert the stoppers in the flasks,invert,and shake briefly.After 1minute,accurately timed,determine the relative fluorescence intensities of both solutions in 1-cm cells,with a suitable fluorometer,at about 485nm,with the excitation wavelength at about 385nm.The fluorescence intensity of the solution obtained from the Test solutionis not greater than the difference between the intensities of the two solutions (not more than 1.0%).
Ordinary impurities á466ñ—
Test solution:
methanol.
Standard solution:
methanol.
Eluant:
a mixture of methanol and ammonium hydroxide (18:1).
Visualization:
16.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Dissolve about 200mg of Ethambutol Hydrochloride,accurately weighed,in a mixture of 100mLof glacial acetic acid and 5mLof mercuric acetate TS.Add crystal violet TS,and titrate with 0.1Nperchloric acid VS(the color change at the endpoint is from blue to blue-green).Perform a blank determination,and make any necessary corrections.Each mLof 0.1Nperchloric acid is equivalent to 13.86mg of C10H24N2O2·2HCl.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 786
Phone Number:1-301-816-8394