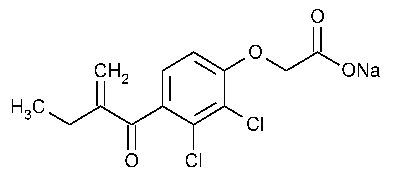
Ethacrynate Sodium for Injection
»Ethacrynate Sodium for Injection is a sterile,freeze-dried powder prepared by the neutralization of Ethacrynic Acid with the aid of Sodium Hydroxide.It contains an amount of ethacrynate sodium equivalent to not less than 90.0percent and not more than 110.0percent of the labeled amount of C13H12Cl2O4.
Labeling—
Label it to indicate that it was prepared by freeze-drying,having been filled into its container in the form of a true solution.
Constituted solution—
At the time of use,it meets the requirements for Constituted Solutionsunder Injections á1ñ.
Identification,Ultraviolet Absorption á197Uñ—
Solution:
50µg per mL.
Medium:
acidified methanol (prepared by adding 9mLof hydrochloric acid to 100mLof methanol).
Bacterial endotoxins á85ñ—
It contains not more than 5.0USP Endotoxin Units per mg of ethacrynate sodium.
pHá791ñ:
between 5.0and 7.0,in a solution containing the equivalent of about 50mg of ethacrynic acid in 50mLof Sterile Water for Injection.
Other requirements—
It meets the requirements for Sterility Tests á71ñand Uniformity of Dosage Units á905ñunder Injections á1ñ.
Assay—
Triethylamine solution,Solvent mixture,Mobile phase,Standard preparation,and Chromatographic system—
Prepare as directed in the Assayunder Ethacrynic Acid Tablets.
Assay preparation—
Select a number of containers of Ethacrynate Sodium for Injection,the combined contents of which,on the basis of the labeled amount,are equivalent to about 500mg of ethacrynic acid.Add about 5mLof Solvent mixtureto each container,mix to dissolve the contents,and combine the resulting solutions in a 200-mLvolumetric flask.Rinse each container with two additional 5-mLportions of Solvent mixture,add the rinsings to the solution in the volumetric flask,dilute with Solvent mixtureto volume,and mix.Transfer 20.0mLof this solution to a 100-mLvolumetric flask,dilute with Solvent mixtureto volume,and mix.
Procedure—
Proceed as directed for Procedurein the Assayunder Ethacrynic Acid Tablets.Calculate the quantity,in mg,of C13H12Cl2O4in each container of Ethacrynate Sodium for Injection taken by the formula:
(1000C/N)(rU/rS),
in which Nis the number of containers selected for the Assay preparation.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 784
Pharmacopeial Forum:Volume No.27(4)Page 2731
Phone Number:1-301-816-8305