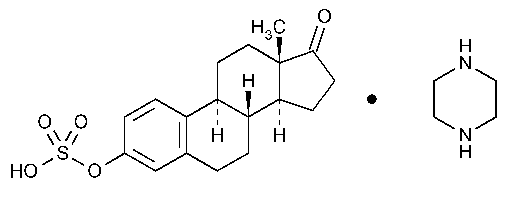
Estropipate
Estra-1,3,5(10)-trien-17-one,3-(sulfooxy)-,compd.with piperazine (1:1).
Estrone hydrogen sulfate compound with piperazine (1:1) [7280-37-7].
»Estropipate contains not less than 97.0percent and not more than 103.0percent of C18H22O5S·C4H10N2,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification,
Infrared Absorption á197Kñ.
Loss on drying á731ñ—
Dry it at 105 for 1hour:it loses not more than 1.0%of its weight.
for 1hour:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.5%.
Free estrone—
Stock impurity standard preparation—
Weigh accurately 25.0mg of USP Estrone RSinto a 100-mLvolumetric flask,dilute with spectrophotometric-grade methanol to volume,and sonicate to achieve complete solution.
Impurity standard preparation—
Weigh accurately 25.0mg of USP Estropipate RSinto a 25-mLvolumetric flask,add 2.0mLof Stock impurity standard preparation,dilute with spectrophotometric-grade methanol to volume,and sonicate to achieve complete solution.
Standard preparation—
Weigh accurately 25.0mg of USP Estropipate RSinto a 25-mLvolumetric flask,dilute with spectrophotometric-grade methanol to volume,and sonicate to achieve complete solution.
Test preparation—
Using a portion of Estropipate,accurately weighed,prepare as directed under Standard preparation.
Mobile phase—
Mix 650mLof 0.025Mpotassium dihydrogen phosphate with 350mLof spectrophotometric-grade acetonitrile.Filter the solution through a membrane filter having a porosity of 1µm or less,and degas at a pressure of less than 100mm of mercury until no further bubbles appear.The concentration of acetonitrile may be varied to meet system suitability requirements and to provide a suitable elution time for all components.
Chromatographic system—
Typically,a high-pressure liquid chromatograph,operated at room temperature,is fitted with a 30-cm ×3.9-mm stainless steel column that contains packing L1.The mobile phase is maintained at a pressure and flow rate (approximately 1.5mLper minute)capable of giving the required resolution (see System suitability test)and a suitable elution time.An UVdetector that monitors absorption at a wavelength of 213nm is used with a recorder adjusted such that approximately 0.04absorbance unit gives a full-scale reading.
System suitability test—
Chromatograph two injections of the Impurity standard preparation,and determine that after the injection front the small peak (estrone)after the major peak does not differ in peak response between the duplicate injections by more than 4%.Also determine that the small peak after the major component has a retention time relative to the major component of approximately 5.5.(For a particular column,resolution may be increased by decreasing the amount of acetonitrile in the Mobile phase.)
Procedure—
Inject separately 5.0-µLportions of the Standard preparation,the Impurity standard preparation,and the Test preparationinto the high-pressure liquid chromatograph by means of a suitable sampling valve or high-pressure microsyringe.Measure the peak responses for the estrone peak relative to the estropipate peak obtained with the Standard preparation,the Impurity standard preparation,and the Test preparation.Calculate the percentage of free estrone taken by the formula:
2.5(C/W)(HU/HS),
in which HUand HSare the measured peak heights of the impurity (estrone)in the Test preparationand the Impurity standard preparationcorrected for the peak height of estrone in the Standard preparation,respectively,Wis the weight,in mg,of estropipate in the Test preparation,and Cis the concentration,in µg per mL,of USP Estrone RSin the Impurity standard preparation.Not more than 2.0%is found.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Assay—
Standard preparation—
Prepare as directed under Free estrone.
Assay preparation—
Prepare as directed for Test preparationunder Free estrone.
Chromatographic system—
Use the same system as in test for Free estrone.Adjust the recorder so that approximately 0.4absorbance unit gives a full-scale reading.
System suitability test—
Chromatograph two injections of the Standard preparation,and determine that only one major peak is observed after the injection front.The peak responses between the duplicate injections for the major peak do not differ by more than 3%.
Procedure—
Inject 5.0µLof the Assay preparationand the Standard preparationinto the high-pressure liquid chromatograph by means of a suitable sampling valve or high-pressure microsyringe.Measure the peak heights for the respective estropipate peak (it is actually an estrone sulfate peak)obtained with the Assay preparationand the Standard preparation.Calculate the quantity,in mg,of C18H22O5S·C4H10N2in the portion of Estropipate taken by the formula:
25C(HU/HS),
in which HUand HSare the peak heights obtained with the Assay preparationand the Standard preparation,respectively,and Cis the concentration,in mg per mL,of USP Estropipate RSin the Standard preparation.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 782
Phone Number:1-301-816-8139