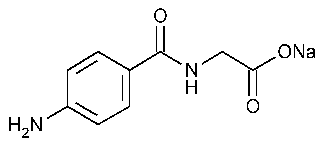
Aminohippurate Sodium Injection
Glycine,N-(4-aminobenzoyl)-,monosodium salt.
Monosodium p-aminohippurate [94-16-6].
»Aminohippurate Sodium Injection is a sterile solution of Aminohippuric Acid in Water for Injection prepared with the aid of Sodium Hydroxide.It contains not less than 95.0percent and not more than 105.0percent of the labeled amount of C9H9N2NaO3.
Packaging and storage—
Preserve in single-dose or multiple-dose containers,preferably of Type Iglass.
Identification—
A:
Avolume of Injection,equivalent to 100mg of aminohippuric acid,diluted to 50mLand acidified with hydrochloric acid,responds to Identificationtest Bunder Aminohippuric Acid.
B:
Transfer a volume of Injection,equivalent to about 200mg of aminohippurate sodium,to a test tube,and add,in the order named,2mLof potassium iodide TS,10mLof water,and 5mLof sodium hypochlorite TS:a red color is produced.
C:
It responds to the flame test for Sodium á191ñ.
Bacterial endotoxins á85ñ—
It contains not more than 0.04USP Endotoxin Unit per mg of aminohippurate sodium.
pHá791ñ:
between 6.7and 7.6.
Other requirements—
It meets the requirements under Injections á1ñ.
Assay—
Transfer to a 200-mLvolumetric flask an accurately measured volume of Injection,equivalent to about 1g of aminohippurate sodium,and dilute with water to volume.Transfer 50.0mLof the solution to a suitable container,add 5mLof hydrochloric acid,and proceed as directed under Nitrite Titration á451ñ,beginning with “cool to about 15 .”Each mLof 0.1Msodium nitrite is equivalent to 21.62mg of C9H9N2NaO3.
.”Each mLof 0.1Msodium nitrite is equivalent to 21.62mg of C9H9N2NaO3.
Auxiliary Information—
Staff Liaison:Larry N.Callahan,Ph.D.,Scientist
Expert Committee:(BNT)Biotechnology and Natural Therapeutics/Diagnostics
USP28–NF23Page 126
Phone Number:1-301-816-8385