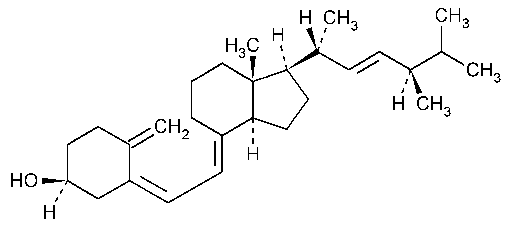
Ergocalciferol
9,10-Secoergosta-5,7,10(19),22-tetraen-3-ol,(3b,5Z,7E,22E)-.
Ergocalciferol [50-14-6].
»Ergocalciferol contains not less than 97.0percent and not more than 103.0percent of C28H44O.
Packaging and storage—
Preserve in hermetically sealed containers under nitrogen,in a cool place and protected from light.
USP Reference standards á11ñ—
USP Ergocalciferol RS.USP Ergosterol RS.USP Vitamin D Assay System Suitability RS.
Identification—
A:
Infrared Absorption á197Kñ(range 2to 12µm).
B:
Ultraviolet Absorption á197Uñ—
Solution:
10µg per mL.
Medium:
alcohol.
Absorptivities at 265nm do not differ by more than 3.0%.
C:
To a solution of about 0.5mg in 5mLof chloroform add 0.3mLof acetic anhydride and 0.1mLof sulfuric acid,and shake vigorously:a bright red color is produced and rapidly changes through violet and blue to green.
D:
Prepare without heating,and handle without delay,a solution of squalane in chloroform (1in 100)containing 50mg of Ergocalciferol per mL,and prepare a Standard solution of USP Ergocalciferol RSin the same solvent and of the same concentration.Prepare a solution of squalane in chloroform (1in 100)containing 100µg of USP Ergosterol RSper mL.Apply 10µLof the test solution,10µLof the Standard solution,and 10µLof the ergosterol solution on a line parallel to and about 2.5cm from the bottom edge of a thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Place the plate in a developing chamber containing,and equilibrated with,a mixture of equal volumes of cyclohexane and ether.Develop the chromatogram until the solvent front has moved about 15cm above the line of application.Perform the development and subsequent operations in the dark.Remove the plate,allow the solvent to evaporate,and spray with a solution of acetyl chloride in antimony trichloride TS(1in 50).The chromatogram obtained with the test solution shows a yellowish orange area (ergocalciferol)having the same RFvalue as the area of the Standard solution of ergocalciferol and may show a violet area below the ergocalciferol area.The color of the violet area is not more intense than that of the violet area in the chromatogram obtained from the solution of ergosterol.
Specific rotation á781Sñ:
between +103 and +106
and +106 .
.
Test solution:
15mg per mL,in alcohol.Prepare the solution without delay,using Ergocalciferol from a container opened not longer than 30minutes,and determine the optical rotation within 30minutes after the solution has been prepared.
Reducing substances—
To 10mLof a solution in dehydrated alcohol (1in 100)add 0.5mLof a solution of blue tetrazolium in methanol (1in 200).Then add 0.5mLof a solution of tetramethylammonium hydroxide TSin dehydrated alcohol (1in 10).Allow the mixture to stand for 5minutes,accurately timed,then add 1mLof glacial acetic acid.Prepare a blank by treating 10mLof dehydrated alcohol in the same manner.Determine the absorbance of the solution at 525nm,with a suitable spectrophotometer,against the blank:the absorbance is not greater than that obtained from a solution containing 0.2µg per mLof hydroquinone in dehydrated alcohol,similarly treated.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent:
dimethyl sulfoxide.
Assay—
Dehydrated hexane
,Mobile phase,Chromatographic system,System suitability preparation,and System suitability test—Proceed as directed in the Assayunder Cholecalciferol.
Standard preparation—
[NOTE—Use low-actinic glassware,and prepare solutions fresh daily.]Transfer about 30mg of USP Ergocalciferol RS,accurately weighed,to a 50-mLvolumetric flask,dissolve without heat in toluene,add toluene to volume,and mix.Pipet 10mLof this stock solution into a 50-mLvolumetric flask,dilute with Mobile phaseto volume,and mix to obtain a solution having a known concentration of about 120µg per mL.
Assay preparation—
[NOTE—Use low-actinic glassware,and prepare solutions fresh daily.]Transfer about 30mg of Ergocalciferol,accurately weighed,to a 50-mLvolumetric flask,and proceed as directed for Standard preparation,beginning with “dissolve without heat in toluene,”to obtain a solution having a concentration of about 120µg per mL.
Procedure—
Introduce equal volumes (5to 10µL)of the Standard preparationand the Assay preparationinto the chromatograph (see Chromatography á621ñ)by means of a suitable sampling valve.Measure the responses for the major peaks obtained,at corresponding retention times,with the Assay preparationand the Standard preparation.Calculate the quantity,in mg,of C28H44Oin the portion of Ergocalciferol taken by the formula:
0.25C(rU/rS),
in which Cis the concentration,in µg per mL,of USP Ergocalciferol RSin the Standard preparation;and rUand rSare the peak responses for ergocalciferol obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(DSN)Dietary Supplements:Non-Botanicals
USP28–NF23Page 745
Phone Number:1-301-816-8389