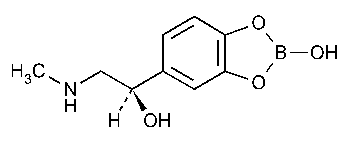
Epinephryl Borate Ophthalmic Solution
1,3,2-Benzodioxaborole-5-methanol,2-hydroxy-a-[(methylamino)methyl]-,(R)-.
(-)-3,4-Dihydroxy-a-[(methylamino)methyl]benzyl alcohol,cyclic 3,4-ester with boric acid [5579-16-8].
»Epinephryl Borate Ophthalmic Solution is a sterile solution in water of Epinephrine as a borate complex.It contains an amount of epinephryl borate (C9H12BNO4)equivalent to not less than 90.0percent and not more than 115.0percent of the labeled amount of epinephrine (C9H13NO3).It contains a suitable antibacterial agent and one or more suitable preservatives and buffering agents.
Packaging and storage—
Preserve in small,well-filled,tight,light-resistant containers.
Labeling—
The label indicates that the Ophthalmic Solution is not to be used if its color is pinkish or darker than slightly yellow or if it contains a precipitate.
Color and clarity—
Standard solution—
Transfer 2.0mLof 0.100Niodine VSto a 500-mLvolumetric flask,dilute with water to volume,and mix.
Procedure—
Visually examine a portion of the Ophthalmic Solution (Test solution)in a suitable clear glass test tube against a white background:it is not pinkish,and it contains no precipitate.If any yellow color is observed in the Test solution,concomitantly determine the absorbances of the Test solutionand the Standard solutionin 1-cm cells with a suitable spectrophotometer set at 460nm:the absorbance of the Test solutiondoes not exceed that of the Standard solution.
Identification—
A:
To 5mLof pH4.0acid phthalate buffer (see Buffer Solutionsin the section Reagents,Indicators,and Solutions)add 0.5mLof Ophthalmic Solution and 1mLof 0.1Niodine.Mix,allow to stand for 5minutes,and add 2mLof 0.1Nsodium thiosulfate:a deep red color is produced.
B:
To 5mLin a porcelain evaporating dish add 5drops of sulfuric acid and 5mLof methanol:the ignited mixture burns with a green-bordered flame.
Sterility á71ñ:
meets the requirements.
pHá791ñ:
between 5.5and 7.6.
Assay—
Transfer an accurately measured volume of Ophthalmic Solution,equivalent to about 100mg of epinephrine,to a 250-mLseparator.Dilute with water to 30mL,and adjust with dilute hydrochloric acid (1in 12)to a pHof 4.0±0.2.Add 25mLof carbon tetrachloride,shake vigorously for 1minute,allow the phases to separate,and discard the carbon tetrachloride washing.In the same manner,wash with two additional 25-mLportions of carbon tetrachloride,and discard the washings.Rinse the stopper and the mouth of the separator with 2to 3mLof water such that the rinsings enter the separator and combine with the solution under assay.Add 0.2mLof starch TS,and,while swirling the separator,add iodine and potassium iodide TSdropwise until the blue color persists.Immediately add a volume of 0.1Nsodium thiosulfate just sufficient to discharge the blue color.[NOTE—Proceed with the assay from this point without delay.]Add 2.10g of sodium bicarbonate through a dry powder funnel to prevent the powder from coming in contact with the mouth of the separator,and swirl to dissolve most of the solid.By means of a syringe fitted with a suitable pipet,rapidly inject 1.0mLof acetic anhydride directly into the contents of the separator.Swirl the unstoppered separator gently for 3minutes to allow carbon dioxide to escape.Insert the stopper,and shake gently until the evolution of carbon dioxide has ceased (7to 10minutes),releasing the pressure through the stopcock as necessary.Allow to stand for 5minutes.Extract with six 25-mLportions of chloroform,shaking for 1minute each time,filtering each extract through a small pledget of chloroform-saturated cotton and collecting the extracts in a 400-mLbeaker.Add several glass beads,and evaporate on a steam bath to about 3mL.With the aid of 15to 20mLof chloroform,transfer the residue to a tared 50-mLbeaker,and evaporate on the steam bath to dryness.Dry the residue at 105 for 30minutes,cool in a desiccator,and weigh the triacetylepinephrine so obtained.Transfer 10.0mLof chloroform to the beaker,and gently swirl to dissolve the residue,dislodging the semisolid residue from the glass surface,if necessary,with a small metal spatula.Determine the angular rotation of the solution in a 100-mm polarimeter tube.Calculate the quantity,in mg,of epinephrine (C9H13NO3)in the volume of Ophthalmic Solution taken by the formula:
for 30minutes,cool in a desiccator,and weigh the triacetylepinephrine so obtained.Transfer 10.0mLof chloroform to the beaker,and gently swirl to dissolve the residue,dislodging the semisolid residue from the glass surface,if necessary,with a small metal spatula.Determine the angular rotation of the solution in a 100-mm polarimeter tube.Calculate the quantity,in mg,of epinephrine (C9H13NO3)in the volume of Ophthalmic Solution taken by the formula:
(183.20/309.32)(W)(0.5+0.5R/93),
in which 183.20and 309.32are the molecular weights of epinephrine and triacetylepinephrine,respectively;Wis the weight,in mg,of the isolated triacetylepinephrine;and Ris the specific rotation,in degrees,of the triacetylepinephrine solution.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(PA6)Pharmaceutical Analysis 6
USP28–NF23Page 744
Pharmacopeial Forum:Volume No.30(4)Page 1192
Phone Number:1-301-816-8389