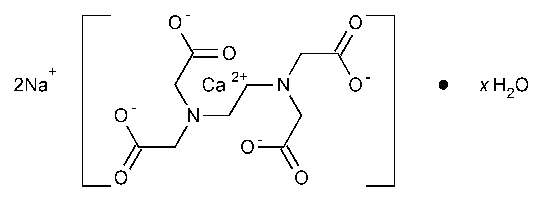
Edetate Calcium Disodium
Calciate(2-),[[N,N¢-1,2-ethanediylbis[N-(carboxymethyl)glycinato]](4-)-N,N¢,O,O¢,ON,ON¢]-,disodium,hydrate,(OC-6-21)-.
Disodium[(ethylenedinitrilo)tetraacetato]calciate(2-)hydrate [23411-34-9].
Anhydrous [62-33-9].
»Edetate Calcium Disodium is a mixture of the dihydrate and trihydrate of calcium disodium ethylenediaminetetraacetate (predominantly the dihydrate).It contains not less than 97.0percent and not more than 102.0percent of C10H12CaN2Na2O8,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
Infrared Absorption á197Mñ.
B:
Asolution (1in 20)responds to the oxalate test for Calcium á191ñand to the flame test for Sodium á191ñ.
C:
To 5mLof water add 2drops of ammonium thiocyanate TSand 2drops of ferric chloride TS.To the deep red solution add about 50mg of Edetate Calcium Disodium,and mix:the deep red color disappears.
pHá791ñ:
between 6.5and 8.0,in a solution (1in 5).
Water,Method Iá921ñ:
not more than 13.0%.
Heavy metals,Method IIá231ñ:
0.002%.
Limit of nitrilotriacetic acid—
Mobile phase,Cupric nitrate solution,Stock standard solution,and Chromatographic system—
Prepare as directed in the test for Limit of nitrilotriacetic acidunder Edetate Disodium.
Resolution solution—
Using Edetate Calcium Disodium instead of Edetate Disodium,prepare as directed for Resolution solutionin the test for Limit of nitrilotriacetic acidunder Edetate Disodium.
Standard preparation—
Transfer 1.0g of Edetate Calcium Disodium to a 100-mLvolumetric flask,add 100µLof Stock standard solution,dilute with Cupric nitrate solutionto volume,and mix.Sonicate,if necessary,to achieve complete solution.
Test preparation—
Transfer 1.0g of Edetate Calcium Disodium to a 100-mLvolumetric flask,dilute with Cupric nitrate solutionto volume,and mix.Sonicate,if necessary,to achieve complete solution.
Procedure—
Proceed as directed for Procedurein the test for Limit of nitrilotriacetic acidunder Edetate Disodium:the response of the nitrilotriacetic acid peak of the Test preparationdoes not exceed the difference between the nitrilotriacetic acid peak responses obtained from the Standard preparationand the Test preparation(0.1%).
Magnesium-chelating substances—
Weigh accurately 1.0g,transfer to a small beaker,and dissolve in 5mLof water.Add 5mLof ammonia-ammonium chloride buffer TS.Then to the buffered solution add 5drops of eriochrome black TS,and titrate with 0.10Mmagnesium acetate to the appearance of a deep wine-red color:not more than 2.0mLis required.
Assay—
Weigh accurately about 1.2g of Edetate Calcium Disodium,transfer to a 250-mLbeaker,and dissolve in 75mLof water.Add 25mLof 1Nacetic acid and 1mLof diphenylcarbazone TS,and titrate slowly with 0.1Mmercuric nitrate VSto the appearance of the first purplish color.Perform a blank determination,and make any necessary correction.Each mLof 0.1Mmercuric nitrate is equivalent to 37.43mg of C10H12CaN2Na2O8.
Auxiliary Information—
Staff Liaison:Elena Gonikberg,Ph.D.,Scientist
Expert Committee:(PA4)Pharmaceutical Analysis 4
USP28–NF23Page 713
Pharmacopeial Forum:Volume No.29(5)Page 1474
Phone Number:1-301-816-8251