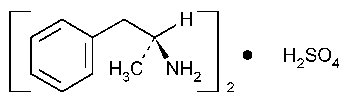Dextroamphetamine Sulfate
Benzeneethanamine,a-methyl-,(S)-,sulfate (2:1).
(+)-a-Methylphenethylamine sulfate (2:1) [51-63-8].
»Dextroamphetamine Sulfate,the dextrorotatory isomer of amphetamine sulfate,contains not less than 98.0percent and not more than 101.0percent of (C9H13N)2·H2SO4,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
Dissolve about 100mg in 5mLof water,add 5mLof 1Nsodium hydroxide,cool to 10 to 15
to 15 ,add 1mLof a mixture of 1volume of benzoyl chloride and 2volumes of anhydrous ethyl ether,insert the stopper,and shake for 3minutes.Filter the precipitate,wash it with about 10mLof cold water,and recrystallize it from diluted alcohol:the crystals of the benzoyl derivative of dextroamphetamine so obtained,after being dried at 105
,add 1mLof a mixture of 1volume of benzoyl chloride and 2volumes of anhydrous ethyl ether,insert the stopper,and shake for 3minutes.Filter the precipitate,wash it with about 10mLof cold water,and recrystallize it from diluted alcohol:the crystals of the benzoyl derivative of dextroamphetamine so obtained,after being dried at 105 for 1hour,melt between 155
for 1hour,melt between 155 and 160
and 160 .
.
B:
Asolution (1in 10)responds to the tests for Sulfate á191ñ.
Specific rotation á781Sñ:
between +20 and +23.5
and +23.5 .
.
Test solution:
40mg per mL,in water.
pHá791ñ:
between 5.0and 6.0,in a solution (1in 20).
Loss on drying á731ñ—
Dry it at 105 for 2hours:it loses not more than 1.0%of its weight.
for 2hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Change to read:
Chromatographic purity—
Diluent—
Dilute 3.12mLof phosphoric acid with water to 1000mL.
Buffer solution—
Dissolve 2.16g of sodium 1-octanesulfonate in 1000mLof water,and add 1.0mLof  triethylamine.
triethylamine. USP28Mix,and adjust with phosphoric acid to a pHof 2.5.
USP28Mix,and adjust with phosphoric acid to a pHof 2.5.
Mobile phase—
Prepare a filtered and degassed mixture ofBuffer solution,acetonitrile,and methanol (144:37:19).Make adjustments if necessary (seeSystem Suitability underChromatography á621ñ).
Standard stock solution—
Dissolve an accurately weighed quantity of USP Dextroamphetamine Sulfate RSinDiluent to obtain a solution having a known concentration of about 0.3mg per mL.
Standard solution—
Dilute an accurately measured volume ofStandard stock solution inDiluent to obtain a solution having a known concentration of about 0.003mg per mL.
Test solution—
Transfer about 30mg of Dextroamphetamine Sulfate,accurately weighed,to a 100-mLvolumetric flask.Dissolve in 50mLofDiluent,sonicate for 5minutes,dilute withDiluent to volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 215-nm detector and a 4.6-mm ×15-cm column that contains 5-µm packing L1.The flow rate is about 1mLper minute.Chromatograph theStandard stock solution,and record the peak responses as directed forProcedure:the tailing factor is not more than 2.0;and the relative standard deviation for replicate injections is not more than 2.0%.Chromatograph theTest solution,and record the peak responses as directed forProcedure:the resolution,R,between the dextroamphetamine peak and any adjacent peak is not less than 1.5.
Procedure—
Separately inject equal volumes (about 50µL)of theStandard solution and theTest solution into the chromatograph,record the chromatograms,and measure the peak responses.Calculate the percentage of each impurity in the portion of Dextroamphetamine Sulfate taken by the formula:
10,000(C/W)(ri/rS),
in which Cis the concentration,in mg per mL,of USP Dextroamphetamine Sulfate RSin theStandard solution;Wis the weight,in mg,of Dextroamphetamine Sulfate taken to prepare theTest solution;riis the peak response for each impurity obtained from theTest solution;and rSis the peak response for amphetamine obtained from theStandard solution:not more than 0.1%of any individual impurity is found;and not more than 0.5%of total impurities is found.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Dissolve about 500mg of Dextroamphetamine Sulfate,accurately weighed,in 50mLof glacial acetic acid,and titrate with 0.1Nperchloric acid VS,determining the endpoint potentiometrically.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 36.85mg of (C9H13N)2·H2SO4.
Auxiliary Information—
Staff Liaison:Salvador C.Salado,M.S.,Scientist and Latin American Liaison
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 606
Pharmacopeial Forum:Volume No.30(3)Page 831
Phone Number:1-301-816-8165