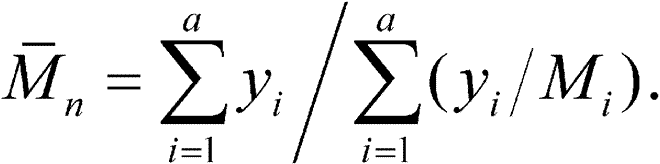Dextran 70
Dextrans.
Dextrans.
»Dextran 70is derived by controlled hydrolysis and fractionation of polysaccharides elaborated by the fermentative action of certain appropriate strains of Leuconostoc mesenteroides(NRRL,B-512F;NCTC,10817)on a sucrose substrate.It is a glucose polymer in which the linkages between glucose units are almost entirely of the a-1:6type.Its weight average molecular weight is in the 63,000to 77,000range.
Packaging and storage—
Preserve in well-closed containers.Store at 25 ,excursions permitted between 15
,excursions permitted between 15 and 30
and 30 .
.
Labeling—
Where it is intended for use in preparing injectable dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference standards á11ñ—
USP Dextran 70RS.USP Dextran 4Calibration RS.USP Dextran 10Calibration RS.USP Dextran 40Calibration RS.USP Dextran 70Calibration RS.USP Dextran 250Calibration RS.USP Dextran VoMarker RS.USP Dextran 70System Suitability RS.USP Endotoxin RS.
Color of solution—
The absorbance of a solution in water (6in 100),measured in a 4-cm cell determined at 375nm against a water blank,is not greater than 0.15.
Identification—
A:Infrared Absorption á197Kñ.
B:
Proceed as directed under Dextran 40,except to use Dextran 70to prepare the Test solutions:the value of the results at the intercept is between 24and 29mLper g.
Specific rotation á781Sñ:
between +195 and +203
and +203 .
.
Test solution:
20mg per mL,heated,if necessary,on a water bath to dissolve.
Bacterial endotoxins á85ñ(where it is labeled as intended for use in the preparation of injectables)—
When tested in Sodium Chloride Injection(0.6in 10),it contains not more than 0.5USP Endotoxin Unit per mL.
Safety—
Inject intravenously 1.0mLof a sterile solution of 6%Dextran 70in saline TSinto each of five mice weighing 18to 20g.The injection period is not less than 10seconds and not greater than 15seconds.If there are no deaths within 72hours,it meets the requirements of the test.If 1or more animals die,continue the test using 10mice weighing 20±0.5g.If all animals survive for 72hours,the requirements of the test are met.
pHá791ñ:
between 4.5and 7.0,in a solution (6in 100).
Loss on drying á731ñ—
Dry it at 105 for 5hours:it loses not more than 7.0%of its weight.
for 5hours:it loses not more than 7.0%of its weight.
Sulfate á221ñ—
A1.5-g portion shows no more sulfate than corresponds to 0.45mLof 0.020Nsulfuric acid (0.03%).
Heavy metals,Method IIá231ñ:
5µg per g.
Limit of nitrogenous impurities (where it is labeled as intended for use in the preparation of injectables)—
Sulfate solutionand Indicator—
Proceed as directed under Dextran 40.
Procedure—
Proceed as directed under Dextran 40,except to use 0.2g of Dextran 70.
Limit of alcohol and related impurities—
Test solution—
Proceed as directed under Dextran 40,except to use 5.0g of Dextran 70.
Standard solution,Chromatographic system,and Procedure—
Proceed as directed under Dextran 40.
Antigenic impurities (where it is labeled as intended for use in the preparation of injectables)—
Prepare a sterile solution containing 60mg per mLof Dextran 70in Sodium Chloride Injection,and proceed as directed under Dextran 40beginning with “At intervals of about 48hours.”
Molecular weight distribution and weight and number average molecular weights—
Mobile phase,Calibration solutions,andMarker solution—
Proceed as directed under Dextran 40.
System suitability solution—
Prepare a solution of USP Dextran 70System Suitability RSin Mobile phasecontaining 20mg per mL.
Test solution—
Prepare a solution of Dextran 70in Mobile phasecontaining 20mg per mL.
Chromatographic system (see Chromatography á621ñ)—
Proceed as directed underDextran 40:Mwfor the total molecular weight distribution is between 65,000and 74,000;Mwfor the high-fraction dextran is between 180,000and 240,000;andMwfor the low-fraction dextran is between 7,000and 11,000.
Procedure—
Chromatograph a 50-µLvolume of the Test solution,and record the peak responses.Calculate values of bar(M)wof the total molecular weight distribution,of the high-fraction dextran,and of the low-fraction dextran as directed for the System suitability solutionunder Chromatographic system.The bar(M)wvalues are between 63,000and 77,000,not more than 195,000,and not less than 13,000,respectively.With the values of b1,b2,b3,b4,and b5,obtained with the Calibration solutionsunder Chromatographic system,calculate the number average molecular weight,bar(M)n,of the total molecular weight distribution of the Test solutionby substituting the corresponding values ofMi,along with their corresponding values of yi,in the equation:
The number average molecular weight,bar(M)n,is between 34,000and 48,000.Where Dextran 70is labeled as intended for use in the preparation of injectables,the ratio bar(M)w/bar(M)nis in the 1.4to 1.9range.
Auxiliary Information—
Staff Liaison:Radhakrishna S Tirumalai,Scientist
Expert Committee:(BBP)Blood and Blood Products
USP28–NF23Page 605
Pharmacopeial Forum:Volume No.29(6)Page 1868
Phone Number:1-301-816-8339