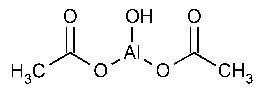
Aluminum Subacetate Topical Solution
Aluminum,bis(acetato-O)hydroxy-.
Bis(acetato)hydroxyaluminum.
Basic aluminum acetate [142-03-0;8000-61-1].
»Aluminum Subacetate Topical Solution yields,from each 100mL,not less than 2.30g and not more than 2.60g of aluminum oxide (Al2O3),and not less than 5.43g and not more than 6.13g of acetic acid (C2H4O2).It may be stabilized by the addition of not more than 0.9percent of boric acid.
Aluminum Subacetate Topical Solution may be prepared as follows.
| Aluminum Sulfate | 145g |
| Acetic Acid | 160mL |
| Calcium Carbonate | 70g |
| Purified Water,a sufficient quantity, | |
| to make | 1000mL |
Dissolve the Aluminum Sulfate in 600mLof cold water,filter the solution,and add the Calcium Carbonate gradually,in several portions,with constant stirring.Then slowly add the Acetic Acid,mix,and set the mixture aside for 24hours.Filter the product with the aid of vacuum if necessary,returning the first portion of the filtrate to the funnel.Wash the magma on the filter with small portions of cold water,until the total filtrate measures 1000mL.
Packaging and storage—
Preserve in tight containers.
Identification—
It responds to the tests for Aluminum á191ñand for Acetate á191ñ.
pHá791ñ:
between 3.8and 4.6.
Limit of boric acid—
Proceed as directed in the test for Limit of boric acidunder Aluminum Acetate Topical Solution.
Assay for aluminum oxide—
Procedure—
Pipet 20mLof Topical Solution into a 250-mLvolumetric flask,add 5mLof hydrochloric acid,dilute with water to volume,and mix.Pipet 25mLof this dilution into a 250-mLbeaker,and proceed as directed for Procedurein the Assay for aluminum oxideunder Aluminum Acetate Topical Solution,beginning with “add,in the order named.”Each mLof 0.05MEdetate disodium titrantis equivalent to 2.549mg of Al2O3.
Assay for acetic acid—
Proceed as directed in the Assay for acetic acidunder Aluminum Acetate Topical Solution.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(PA6)Pharmaceutical Analysis 6
USP28–NF23Page 96
Phone Number:1-301-816-8389