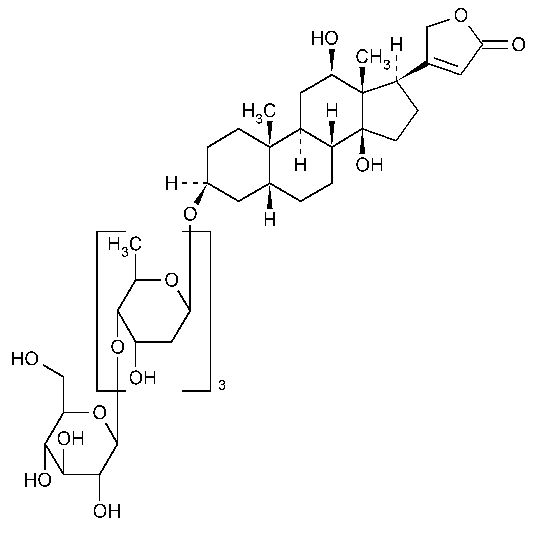
Deslanoside
Card-20(22)-enolide,3-[(O-b-D-glucopyranosyl-(1®4)-O-2,6-dideoxy-b-D-ribo-hexopyranosyl-(1®4)-O-2,6-dideoxy-b-D-ribo-hexopyranosyl-(1®4)-2,6-dideoxy-b-D-ribo-hexopyranosyl)oxy]-12,14-dihydroxy-,(3b,5b,12b)-.
Deacetyllanatoside C [17598-65-1].
»Deslanoside contains not less than 95.0percent and not more than 103.0percent of C47H74O19,calculated on the dried basis.
Caution—Handle Deslanoside with exceptional care,since it is highly potent.
Packaging and storage—
Preserve in tight,light-resistant containers.Store at 25 ,excursions permitted between 15
,excursions permitted between 15 and 30
and 30 .
.
Add the following:
Labeling—
Where it is intended for use in preparing injectable dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification—
Prepare a solution of it in methanol containing about 4mg per mL.Apply 5µLof this solution and 5µLof a methanol solution of USP Deslanoside RScontaining 4mg per mLto a suitable thin-layer chromatographic plate coated with a 0.25-mm layer of chromatographic silica gel.Allow the spots to dry,and develop the chromatogram in a solvent system consisting of a mixture of methylene chloride,methanol,and water (130:36:3)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,and allow the solvent to evaporate.Locate the spots on the plate by lightly spraying with dilute perchloric acid (1in 20)and heating at about 100 for 3minutes.Cool,and examine under UVlight:the RFvalue of the principal spot obtained from the test solution corresponds to that obtained from the Standard solution.
for 3minutes.Cool,and examine under UVlight:the RFvalue of the principal spot obtained from the test solution corresponds to that obtained from the Standard solution.
Specific rotation á781Sñ:
between +7.0 and +8.5
and +8.5 .
.
Test solution:
20mg per mL,in anhydrous pyridine.
Loss on drying á731ñ—
Dry 0.5g in vacuum at 100 to constant weight:it loses not more than 5.0%of its weight.
to constant weight:it loses not more than 5.0%of its weight.
Residue on ignition á281ñ:
not more than 0.2%.
Add the following:
Other requirements—
Where the label states that Deslanoside is sterile,it meets the requirements for Sterility under Deslanoside Injection.
Assay—
Standard preparation—
Dissolve a suitable quantity of USP Deslanoside RSin alcohol,and quantitatively dilute with alcohol to obtain a solution having a known concentration of about 200µg per mL.
Assay preparation—
Transfer about 20mg of Deslanoside,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with alcohol to volume,and mix.
Procedure—
Transfer 3.0mLeach of the Standard preparation,the Assay preparation,and alcohol to provide the blank,to separate 25-mLconical flasks.Evaporate each with gentle warming and with the aid of a current of air just to dryness,and cool in a vacuum desiccator for 30minutes.Add 15.0mLof acid-ferric chloride TSto each flask,mix by swirling,and allow the mixtures to stand protected from light,swirling frequently,at a temperature not exceeding 30 ,for 15minutes.Pass each through separate fine glass wool filters.Concomitantly determine the absorbances of the solutions in 1-cm cells at the wavelength of maximum absorbance at about 590nm,with a suitable spectrophotometer,using the blank to set the instrument.Repeat the measurements at 2-minute intervals until maximum absorbance readings have been obtained.Calculate the quantity,in mg,of C47H74O19in the Deslanoside taken by the formula:
,for 15minutes.Pass each through separate fine glass wool filters.Concomitantly determine the absorbances of the solutions in 1-cm cells at the wavelength of maximum absorbance at about 590nm,with a suitable spectrophotometer,using the blank to set the instrument.Repeat the measurements at 2-minute intervals until maximum absorbance readings have been obtained.Calculate the quantity,in mg,of C47H74O19in the Deslanoside taken by the formula:
0.1C(AU/AS),
in which Cis the concentration,in µg per mL,of USP Deslanoside RSin the Standard preparation;and AUand ASare the maximum absorbances of the solutions from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 582
Pharmacopeial Forum:Volume No.29(5)Page 1448
Phone Number:1-301-816-8305