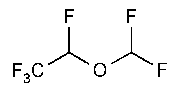
Desflurane
Ethane,2-(difluoromethoxy)-1,1,1,2-tetrafluoro-,(±)-.
(±)-2-Difluoromethyl 1,2,2,2-tetrafluoroethyl ether [57041-67-5].
»Desflurane contains not less than 98.0percent and not more than 102.0percent of C3H2F6O.
Change to read:
Packaging and storage—
Preserve in tight,light-resistant containers.
Change to read:
USP Reference standards á11ñ—
USP Desflurane RS.USP Desflurane Related Compound A RS.
Identification—
The IRabsorption spectrum of it using a gas cell exhibits maxima only at the same wavelengths as that of a similar preparation of USP Desflurane RS.
Limit of nonvolatile residue—
Transfer 10.0mLof it to an accurately weighed evaporating dish,evaporate with a stream of nitrogen to dryness,and dry the residue at 50 for 2hours:the weight of the residue does not exceed 7.5mg (0.075%).
for 2hours:the weight of the residue does not exceed 7.5mg (0.075%).
Limit of antimony—
Diluent A—
Prepare a mixture of nitric acid and water (1:1).
Diluent B—
Prepare a mixture of nitric acid and hydrochloric acid (9:1).
Standard solutions—
Transfer about 0.1mL(234mg)of antimony pentachloride,accurately weighed,to a 50-mLvolumetric flask,dilute with Diluent Bto volume,and mix.This stock solution contains about 1906µg of antimony per mL.Dilute a portion of this solution quantitatively and stepwise with Diluent Bto obtain Standard solutionscontaining 2.5,5.0,and 10.0µg of antimony per mL.
Test solution—
Accurately weigh a stoppered stock bottle containing a quantity of Desflurane at ambient temperature,and then cool it in powdered dry ice.Using a cold syringe,transfer between 5and 7mLof Desflurane from the cold bottle to a separator containing 20mLof Diluent A.Allow the stock bottle containing the remaining Desflurane to come to ambient temperature,weigh it accurately,and calculate the quantity,in g,of Desflurane taken for the test.Allow the Desflurane in the separator to evaporate,and with the aid of a few mLof Diluent Atransfer the acid solution to a clean,dry beaker.Add 1mLof hydrochloric acid to the solution in the beaker,and reduce the volume to about 8mLby evaporating on a hot plate.Transfer this solution to a 10-mLvolumetric flask,dilute with Diluent Bto volume,and mix.
Procedure—
Concomitantly determine the absorbances of the Standard solutionsand the Test solutionat the antimony emission line at 217.6nm with an atomic absorption spectrophotometer (see Spectrophotometry and Light-scattering á851ñ)equipped with an antimony hollow-cathode lamp and an air–acetylene flame,using Diluent Bas the blank.Plot the absorbances of the Standard solutionsversus concentration,in µg per mL,of antimony,and draw the straight line best fitting the three plotted points.From the graph so obtained,determine the concentration,C,in µg per mL,of antimony in the Test solution.Calculate the quantity,in ppm,of antimony in the portion of Desflurane taken by the formula:
10C/W,
in which Wis the quantity,in g,of Desflurane taken to prepare the Test solution.Not more than 3µg per g is found.
Limit of fluoride—
[NOTE—Store all solutions,except the Buffer solution,in plastic containers.]
Buffer solution—
Dissolve 57mLof glacial acetic acid,58g of sodium chloride,and 4g of (1,2-cyclohexylenedinitrilo)tetraacetic acid in 500mLof water.Adjust with 5Nsodium hydroxide to a pHof 5.25±0.25,dilute with water to 1000mL,and mix.
Standard solutions—
Quantitatively dissolve an accurately weighed quantity of USP Sodium Fluoride RSin water to obtain a solution containing 2210µg per mL.Each mLof this solution contains 1000µg of fluoride per mL.Dilute accurately measured volumes of this stock solution with Buffer solutionto obtain solutions having concentrations of 0.1,0.3,0.5,1.0,3.0,and 5.0µg of fluoride per mL.
Test solution—
Transfer 10.0mLof Desflurane to a 60-mLseparator,add 10.0mLof water,shake for 1minute,and allow the layers to separate.Drain the lower organic layer and a small portion of the aqueous layer into a beaker,and discard.Transfer 5.0mLof the aqueous phase remaining in the separator to a plastic cup,add 5.0mLof Buffer solution,and mix.[NOTE—Transfer the aqueous phase remaining in the separator to a second plastic cup,and reserve for the pHdetermination.]
Procedure—
Concomitantly measure the potentials (see pHá791ñ),in mV,of the Standard solutionsand the Test solutionwith a pHmeter capable of a minimum reproducibility of ±0.2mVand equipped with a fluoride-specific ion-indicating electrode and a calomel reference electrode.[NOTE—When taking measurements,immerse the electrodes in the solution,stir with a polytef-coated stirring bar and a magnetic stirrer having an insulated top until equilibrium is attained (about 1to 2minutes),and record the potential.Rinse the electrodes with Buffer solution,and dry,taking care to avoid damaging the crystal of the specific-ion electrode.]Plot the logarithms of the fluoride concentrations,in µg per mL,of the Standard solutionsversus potential,in mV.From the measured potential of the Test solutionand the standard response line,determine the concentration,C,in µg per mL,of fluoride in the Test solution.Multiply Cby 0.0002to obtain the percentage of fluoride in the Desflurane taken.Not more than 0.001%is found.
Related compounds—
Standard solution—
Insert a rubber-sleeve stopper into a 2-mLvial,weigh accurately,and partially evacuate the vial.Using a cold syringe,inject about 680µLof USP Desflurane RS,previously cooled to 0 to 5
to 5 ,into the vial.Allow the vial to come to ambient temperature,accurately weigh it,and calculate the quantity,in mg,of USP Desflurane RSadded.Using a syringe,inject about 7µLof USP Desflurane Related Compound A RSinto the same vial,accurately weigh,and calculate the quantity,in mg,of USP Desflurane Related Compound A RSadded.Similarly,and in turn,add about 7.2µLof trichlorofluoromethane,7.4µLof dichlorofluoromethane,7.6µLof methylene chloride,6.8µLof chloroform,6.3µLof trichlorotrifluoroethane,and 6.7µLof isoflurane,accurately weighing the vial after each addition,and calculating the quantity,in mg,of each addition.Calculate the percentage,PS,of each impurity in the Standard solutiontaken by the formula:
,into the vial.Allow the vial to come to ambient temperature,accurately weigh it,and calculate the quantity,in mg,of USP Desflurane RSadded.Using a syringe,inject about 7µLof USP Desflurane Related Compound A RSinto the same vial,accurately weigh,and calculate the quantity,in mg,of USP Desflurane Related Compound A RSadded.Similarly,and in turn,add about 7.2µLof trichlorofluoromethane,7.4µLof dichlorofluoromethane,7.6µLof methylene chloride,6.8µLof chloroform,6.3µLof trichlorotrifluoroethane,and 6.7µLof isoflurane,accurately weighing the vial after each addition,and calculating the quantity,in mg,of each addition.Calculate the percentage,PS,of each impurity in the Standard solutiontaken by the formula:
100Wi/(WD+Wt),
in which Wiis the quantity,in mg,of the respective impurity added during the preparation of the Standard solution;WDis the quantity,in mg,of USP Desflurane RSin the Standard solution;and Wtis the sum of all the impurities added during the preparation of the Standard solution.
Test solution—
Use Desflurane.
Chromatographic system
(see Chromatography á621ñ)—The gas chromatograph is equipped with a flame-ionization detector and a 2.4-mm ×6.1-m stainless steel column packed with 25%phase G16on 80-to 100-mesh support S1A.Helium is used as the carrier gas at a flow rate of about 20mLper minute.The column temperature is maintained at about 75 ,the injection port temperature at about 200
,the injection port temperature at about 200 ,and the detector temperature at about 250
,and the detector temperature at about 250 .Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.7for trichlorofluoromethane,0.86for bis-(1,2,2,2-tetrafluoroethyl)ether (desflurane related compound A),1.0for desflurane,1.7for dichlorofluoromethane,2.6for isoflurane,3.7for methylene chloride,and 6.6for chloroform;the resolution,R,between trichlorofluoromethane and desflurane related compound Ais not less than 1.2;and the tailing factor for the desflurane related compound Apeak is not more than 1.5.
.Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.7for trichlorofluoromethane,0.86for bis-(1,2,2,2-tetrafluoroethyl)ether (desflurane related compound A),1.0for desflurane,1.7for dichlorofluoromethane,2.6for isoflurane,3.7for methylene chloride,and 6.6for chloroform;the resolution,R,between trichlorofluoromethane and desflurane related compound Ais not less than 1.2;and the tailing factor for the desflurane related compound Apeak is not more than 1.5.
Procedure—
Separately inject 1µLof the Standard solutionand 2µLof the Test solutioninto the chromatograph,record the chromatograms for 40minutes,and measure the area responses for all the peaks.Calculate the percentage of each impurity taken by the formula:
2PS(ri/rS),
in which PSis the percentage of the respective impurity in the Standard solution;riis the peak response of the respective impurity in the chromatogram obtained from the Test solution;and rSis the response of the respective impurity peak in the chromatogram obtained from the Standard solution.Not more than 1.0%of isoflurane,0.1%of methylene chloride,0.1%of desflurane related compound A,0.05%of chloroform,0.025%of trichlorotrifluoroethane,0.01%of dichlorofluoromethane,and 0.01%of trichlorofluoromethane are found.
Change to read:
Assay—
Internal standard solution—
Transfer 2.0mLof  USP Halothane RS
USP Halothane RS USP28to a 100-mLvolumetric flask,dilute with p-xylene to volume,and mix.
USP28to a 100-mLvolumetric flask,dilute with p-xylene to volume,and mix.
Standard preparation—
Transfer 1.0mLof Internal standard solutionto a 2.0-mLseptum-capped vial,cap,seal,and weigh accurately.Using a cold syringe,inject about 25µLof USP Desflurane RS,previously cooled to 0 to 5
to 5 ,into the vial.Allow the vial to come to ambient temperature,accurately weigh it,and calculate the quantity,in mg,of USP Desflurane RSadded.
,into the vial.Allow the vial to come to ambient temperature,accurately weigh it,and calculate the quantity,in mg,of USP Desflurane RSadded.
Assay preparation—
Transfer 1.0mLof Internal standard solutionto a 2.0-mLseptum-capped vial,cap,seal,and weigh accurately.Using a cold syringe,inject about 25µLof Desflurane,previously cooled to 0 to 5
to 5 ,into the vial.Allow the vial to come to ambient temperature,accurately weigh it,and calculate the quantity,in mg,of Desflurane added.
,into the vial.Allow the vial to come to ambient temperature,accurately weigh it,and calculate the quantity,in mg,of Desflurane added.
Chromatographic system(see Chromatography á621ñ)—
The gas chromatograph is equipped with a flame-ionization detector and a 2.4-mm ×3.7-m stainless steel column coated with polytef and packed with 10%phase G31and 15%phase G18on 80-to 100-mesh support S1A.Helium is used as the carrier gas at a flow rate of about 24mLper minute.The chromatograph is programmed as follows.Initially the temperature of the column is maintained at about 80 for 2.5minutes,then increased at a rate of 2
for 2.5minutes,then increased at a rate of 2 per minute,to 88
per minute,to 88 ,maintained at 88
,maintained at 88 for 3minutes,then increased to 175
for 3minutes,then increased to 175 at a rate of 70
at a rate of 70 per minute,and maintained at 175
per minute,and maintained at 175 for 4minutes.The injection port temperature is maintained at about 200
for 4minutes.The injection port temperature is maintained at about 200 ,and the detector temperature at about 250
,and the detector temperature at about 250 .Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative retention times are 1.0for desflurane and about 2.8for halothane;the resolution,R,between desflurane and halothane is not less than 8;and the relative standard deviation for the ratios of the desflurane peak response to the halothane peak response obtained for replicate injections is not more than 1.0%.
.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative retention times are 1.0for desflurane and about 2.8for halothane;the resolution,R,between desflurane and halothane is not less than 8;and the relative standard deviation for the ratios of the desflurane peak response to the halothane peak response obtained for replicate injections is not more than 1.0%.
Procedure—
Separately inject equal volumes (about 1µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the peak area responses for the halothane and desflurane peaks.Calculate the percentage of C3H2F6Oin the Desflurane taken by the formula:
100(WS/WU)(RU/RS),
in which WSis the quantity,in mg,of USP Desflurane RSused to prepare the Standard preparation;WUis the quantity,in mg,of Desflurane used to prepare the Assay preparation;and RUand RSare the ratios of the peak area responses of desflurane to that of halothane obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 579
Pharmacopeial Forum:Volume No.30(4)Page 1187
Phone Number:1-301-816-8379