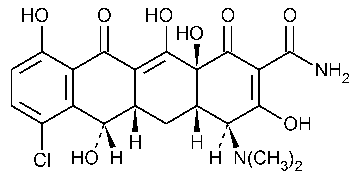
Demeclocycline
2-Naphthacenecarboxamide,7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-1,11-dioxo-,[4S-(4a,4aa,5aa,6b,12aa)]-.
7-Chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-1,11-dioxo-2-naphthacenecarboxamide [127-33-3].
Sesquihydrate 491.88 [13215-10-6].
»Demeclocycline has a potency equivalent to not less than 970µg of demeclocycline hydrochloride (C21H21ClN2O8·HCl)per mg,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
A:
Transfer about 40mg,accurately weighed,to a 250-mLvolumetric flask,add 2mLof 0.1Nhydrochloric acid to dissolve,dilute with water to volume,and mix.Transfer 10.0mLof this solution to a 100-mLvolumetric flask,add about 75mLof water and 5.0mLof 5Nsodium hydroxide,dilute with water to volume,and mix:the UVabsorption spectrum of this solution,measured at 6minutes,accurately timed,after the addition of the sodium hydroxide,exhibits maxima and minima at the same wavelengths as that of a similar solution of USP Demeclocycline Hydrochloride RS,concomitantly measured,and the absorptivity,calculated on the anhydrous basis,at the wavelength of maximum absorbance at about 380nm is between 103.5%and 111.3%of that of the USP Demeclocycline Hydrochloride RS,the potency of the Reference Standard being taken into account.
B:
Transfer 40mg,accurately weighed,to a 200-mLvolumetric flask,add 100mLof 0.1Nhydrochloric acid,shake to dissolve,dilute with 0.1Nhydrochloric acid to volume,and mix.Transfer 5.0mLof this solution into each of two 50-mLvolumetric flasks (Solutions 1and 2).Prepare similar solutions of USP Demeclocycline Hydrochloride RS(Solutions 3and 4).To Solutions 1and 3,add 10mLof 6Nhydrochloric acid,and to Solutions 2and 4,add 10mLof 3Nhydrochloric acid.Heat the four flasks in a water bath for 20minutes,cool,dilute the contents with water to volume,and mix.Determine the absorbances of Solutions 1and 3at the wavelength of maximum absorbance at about 430nm,with a suitable spectrophotometer,using Solutions 2and 4,respectively,as the blanks.Determine the absorbances of Solutions 2and 4at the wavelength of maximum absorbance at about 368nm,using Solutions 1and 3,respectively,as the blanks.Calculate the ratio:
(WSP/1000)(A368+A430)U/WU(A368+A430)S,
in which WSis the weight,in mg,of USP Demeclocycline Hydrochloride RStaken,calculated on the dried basis;Pis the potency,in µg per mg,of the USP Demeclocycline Hydrochloride RS;WUis the weight,in mg,of specimen taken,calculated on the anhydrous basis;and the final two parenthetic expressions are the absorbances of the four solutions at the wavelengths indicated by the subscripts for the specimen (U)and the Standard (S):the ratio is between 0.97and 1.17.
Crystallinity á695ñ:
meets the requirements.
pHá791ñ:
between 4.0and 5.5,in a solution containing 10mg per mL.
Water,Method Iá921ñ:
between 4.3%and 6.7%.
Assay—
Mobile phase—
Transfer 80g of tertiary butyl alcohol to a 1000-mLvolumetric flask with the aid of 200mLof water,add 100mLof 0.2MpH9.0phosphate buffer (prepared by mixing appropriate volumes of 0.2Mdibasic potassium phosphate and 0.2Mmonobasic potassium phosphate),150mLof 0.02Mtetrabutylammonium hydrogen sulfate (adjusted with sodium hydroxide TSto a pHof 9.0),and 100mLof 0.01Medetate disodium (adjusted with sodium hydroxide TSto a pHof 9.0).Dilute with water to volume,mix,and degas.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).Decreasing the amount of tertiary butyl alcohol increases the resolution.
Standard preparation—
Dissolve an accurately weighed quantity of USP Demeclocycline Hydrochloride RSin 0.01Nhydrochloric acid to obtain a solution having a known concentration of about 1mg per mL.
Assay preparation—
Transfer about 45mg of Demeclocycline,accurately weighed,to a 50-mLvolumetric flask,dissolve in 0.01Nhydrochloric acid,dilute with 0.01Nhydrochloric acid to volume,and mix.
Resolution solution—
Prepare a solution of USP Demeclocycline Hydrochloride RSin 0.01Nhydrochloric acid,and allow to stand for 3hours.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm ×25-cm column containing packing L21and maintained at 60±0.5 .The flow rate is about 1mLper minute.Chromatograph the Resolution solution,and record the responses as directed for Procedure:the relative retention times are about 0.7for epidemethylchlortetracycline and 1.0for demeclocycline;and the resolution between the epidemethylchlortetracycline peak and the demeclocycline peak is not less than 3.0.Chromatograph the Standard preparation,and record the responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 2.0%.
.The flow rate is about 1mLper minute.Chromatograph the Resolution solution,and record the responses as directed for Procedure:the relative retention times are about 0.7for epidemethylchlortetracycline and 1.0for demeclocycline;and the resolution between the epidemethylchlortetracycline peak and the demeclocycline peak is not less than 3.0.Chromatograph the Standard preparation,and record the responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
[NOTE—Use peak areas where peak responses are indicated.]Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in µg,of demeclocycline hydrochloride (C21H21ClN2O8·HCl)equivalent in each mg of the Demeclocycline taken by the formula:
50(CE/W)(rU/rS),
in which Cis the concentration,in mg per mL,of USP Demeclocycline Hydrochloride RSin the Standard preparation;Eis the demeclocycline hydrochloride equivalent,in µg per mg,of USP Demeclocycline Hydrochloride RS;Wis the quantity,in mg,of the Demeclocycline taken;and rUand rSare the demeclocycline peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 577
Phone Number:1-301-816-8335