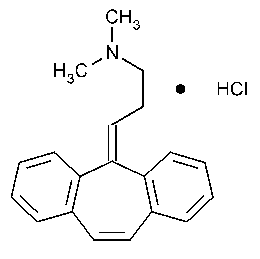
Cyclobenzaprine Hydrochloride
1-Propanamine,3-(5H-dibenzo[a,d]cyclohepten-5-ylidene)-N,N-dimethyl-,hydrochloride.
N,N-Dimethyl-5H-dibenzo[a,d]cycloheptene-D5,g-propylamine hydrochloride [6202-23-9].
»Cyclobenzaprine Hydrochloride contains not less than 99.0percent and not more than 101.0percent of C20H21N·HCl,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:Infrared Absorption á197Mñ.
B:Ultraviolet Absorption á197Uñ—
Solution:
15µg per mL.
Medium:
methanol.
Absorptivities at 290nm,calculated on the dried basis,do not differ by more than 3.0%.
C:
Asolution (1in 50)responds to the tests for Chloride á191ñ.
Melting range á741ñ:
between 215 and 219
and 219 ,but the range between beginning and end of melting does not exceed 2
,but the range between beginning and end of melting does not exceed 2 .
.
Loss on drying á731ñ—
Dry it at 105 to constant weight:it loses not more than 1.0%of its weight.
to constant weight:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.001%.
Chromatographic purity—
Dissolve 100mg in methanol,and dilute with methanol to 5.0mLto obtain the Test solution.Dissolve a suitable quantity of USP Cyclobenzaprine Hydrochloride RSin methanol to obtain a Standard solutionhaving a known concentration of about 20mg per mL.Dilute a portion of this solution quantitatively and stepwise with methanol to obtain a Diluted standard solutionhaving a concentration of about 100µg per mL.Apply separate 5-µLportions of the three solutions to the starting line of a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture and previously washed with methanol.Develop the chromatogram in a suitable chamber with a freshly prepared solvent system consisting of a mixture of acetone,toluene,and ammonium hydroxide (75:25:1)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the chamber,air-dry,and view under short-wavelength UVlight:the RFvalue of the principal spot from the Test solutioncorresponds to that of the Standard solution;and any other spot obtained from the Test solutiondoes not exceed,in size or intensity,the principal spot obtained from the Diluted standard solution(0.5%).
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Test solution:
200mg per mL.
Standard solution—
Proceed as directed except to use 100.0µg of methylene chloride,12.0µg of chloroform,76.0µg of 1,4-dioxane,and 16.0µg of trichloroethylene.
Assay—
Dissolve about 400mg of Cyclobenzaprine Hydrochloride,accurately weighed,in about 80mLof glacial acetic acid,add 15mLof mercuric acetate TS,and titrate with 0.1Nperchloric acid VS,determining the endpoint potentiometrically,using a platinum ring electrode and a sleeve-type calomel electrode containing 0.1Nlithium perchlorate in glacial acetic acid (see Titrimetry á541ñ).Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 31.19mg of C20H21N·HCl.
Auxiliary Information—
Staff Liaison:Salvador C.Salado,M.S.,Scientist and Latin American Liaison
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 556
Pharmacopeial Forum:Volume No.29(4)Page 1024
Phone Number:1-301-816-8165