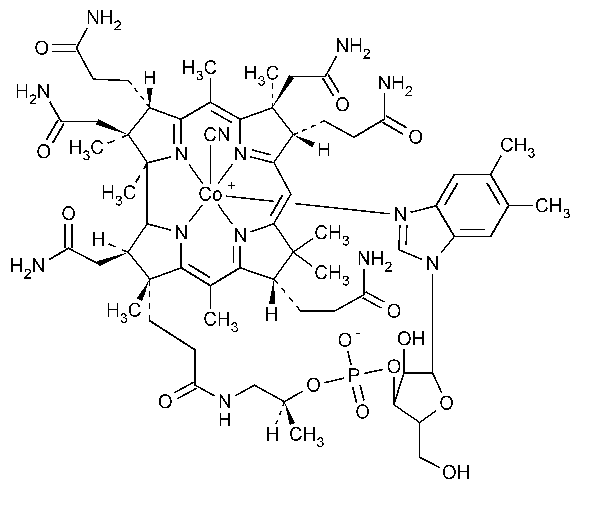
Cyanocobalamin
»Cyanocobalamin contains not less than 96.0percent and not more than 100.5percent of C63H88CoN14O14P,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers,and store at controlled room temperature.
Identification—
A:
The absorption spectrum of the solution employed for measurement of absorbance in the Assayexhibits maxima within ±1nm at 278nm and 361nm and within ±2nm at 550nm.The ratio A361/A278is between 1.70and 1.90,and the ratio A361/A550is between 3.15and 3.40.
B:
Fuse about 1mg with about 50mg of potassium pyrosulfate in a porcelain crucible.Cool,break up the mass with a glass rod,add 3mLof water,and dissolve by boiling.Add 1drop of phenolphthalein TS,and add sodium hydroxide solution (1in 10),dropwise,until just pink.Add 500mg of sodium acetate,0.5mLof 1Nacetic acid,and 0.5mLof nitroso Rsalt solution (1in 500):a red or orange-red color appears at once.Add 0.5mLof hydrochloric acid,and boil for 1minute:the red color persists.
C:
Dissolve about 5mg in 5mLof water in a 50-mLdistilling flask connected with a short,water-cooled condenser.To the flask add 2.5mLof hypophosphorous acid,close,boil gently but short of distillation for 10minutes,then distill 1mLinto a test tube containing 1mLof sodium hydroxide solution (1in 50).To the test tube add 4drops of cold saturated ferrous ammonium sulfate solution,shake gently,then add about 30mg of sodium fluoride,and bring the contents to a boil.Immediately add,dropwise,5Nsulfuric acid until a clear solution results,then add 3to 5drops more of the acid:a blue or blue-green color develops within a few minutes.
Loss on drying á731ñ—
Heat about 25mg,accurately weighed,in a suitable vacuum drying apparatus at 105 and at a pressure of not more than 5mm of mercury for 2hours,cool,and weigh:it loses not more than 12.0%of its weight.
and at a pressure of not more than 5mm of mercury for 2hours,cool,and weigh:it loses not more than 12.0%of its weight.
Pseudo cyanocobalamin—
Dissolve 1.0mg in 20mLof water contained in a small separator,add 5mLof a mixture of equal volumes of carbon tetrachloride and cresol,and shake well for about 1minute.Allow to separate,draw off the lower layer into a second small separator,add 5mLof 5Nsulfuric acid,shake well,and allow to separate completely (the complete separation of the layer may be facilitated by centrifuging):the separated upper layer is colorless or has no more color than a mixture of 0.15mLof 0.10Npotassium permanganate and 250mLof water.
Assay—
Transfer about 30mg of Cyanocobalamin,accurately weighed,with the aid of water to a 1-Lvolumetric flask,dilute with water to volume,and mix.Dissolve an accurately weighed quantity of USP Cyanocobalamin RSin water,and dilute quantitatively and stepwise with water to obtain a Standard solution having a known concentration of about 30µg per mL.Concomitantly determine the absorbances of both solutions in 1-cm cells at the wavelength of maximum absorbance at about 361nm,with a suitable spectrophotometer,using water as the blank.Calculate the quantity,in mg,of C63H88CoN14O14Pin the Cyanocobalamin taken by the formula:
C(AU/AS),
in which Cis the concentration,in µg per mL,of USP Cyanocobalamin RSin the Standard solution;and AUand ASare the absorbances of the solution of Cyanocobalamin and the Standard solution,respectively.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(DSN)Dietary Supplements:Non-Botanicals
USP28–NF23Page 554
Pharmacopeial Forum:Volume No.29(6)Page 1865
Phone Number:1-301-816-8389