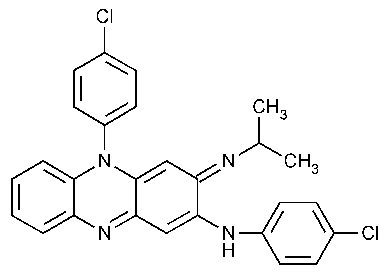
Clofazimine
2-Phenazinamine,N,5-bis(4-chlorophenyl)-3,5-dihydro-3-[(1-methylethyl)imino]-.
3-(p-Chloroanilino)-10-(p-chlorophenyl)-2,10-dihydro-2-(isopropylimino)phenazine [2030-63-9].
»Clofazimine contains not less than 98.5percent and not more than 101.5percent of C27H22Cl2N4,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers,at room temperature.
Identification—
A:
Infrared Absorption á197Sñ:5%solution in methylene chloride.
B:
The RFvalue of the principal spot observed in the chromatogram of the Test preparationcorresponds to that of Standard preparation Aas obtained in the test for Chromatographic purity.
Loss on drying á731ñ—
Dry it at 105 for 3hours:it loses not more than 0.5%of its weight.
for 3hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ—
not more than 0.1%.
Chromatographic purity—
Standard preparations—
Dissolve an accurately weighed quantity of USP Clofazimine RSin methylene chloride,and mix to obtain Standard preparation Ahaving a known concentration of about 0.5mg per mL.Dilute portions of Standard preparation Aquantitatively with methylene chloride to obtain Standard preparations Band Chaving known concentrations of 0.25and 0.1mg per mL,respectively.
Test preparation—
Dissolve an accurately weighed quantity of Clofazimine in methylene chloride to obtain a solution having a known concentration of about 50mg per mL.
Ammonia solution—
Pipet 1mLof ammonium hydroxide into a 100-mLvolumetric flask,dilute with water to volume,and mix.[NOTE—Prepare this solution fresh daily.]
Chromatographic plate—
Use a thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Immediately before use,expose the plate to ammonia vapors for 30minutes by suspending the plate in a tank containing a shallow layer of approximately 25mLof Ammonia solution.[NOTE—Prevent the plate from coming into contact with the liquid.]
Procedure—
Separately apply 5µLof the Test preparationand 5µLof each Standard preparationto the Chromatographic plate.Allow the spots to dry,and develop the chromatogram in a solvent system consisting of a mixture of methylene chloride and n-propyl alcohol (10:1)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,and air-dry.Examine the plate under short-wavelength UVlight,and compare the intensities of any secondary spots observed in the chromatogram of the Test preparationwith those of the principal spots in the chromatograms of the Standard preparations:no secondary spot from the chromatogram of the Test preparationis larger or more intense than the principal spot obtained from Standard preparation A(1.0%),and the sum of the intensities of the secondary spots obtained from the Test preparationcorresponds to not more than 2.0%.
Assay—
Dissolve about 300mg of Clofazimine,accurately weighed,in 5mLof chloroform,with the aid of heat if necessary.Add 20mLof acetone and 5mLof glacial acetic acid,and titrate with 0.1Nperchloric acid VSin glacial acetic acid,determining the endpoint potentiometrically,using a glass electrode and a calomel electrode with a saturated solution of potassium chloride as the bridge fluid and agar gel as the bridge.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 47.34mg of C27H22Cl2N4.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 505
Phone Number:1-301-816-8394