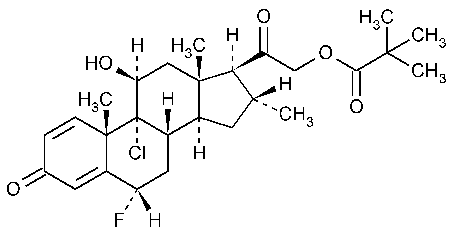
Clocortolone Pivalate
Pregna-1,4-diene-3,20-dione,9-chloro-21-(2,2-dimethyl-1-oxopropoxy)-6-fluoro-11-hydroxy-16-methyl-,(6a,11b,16a)-.
9-Chloro-6a-fluoro-11b,21-dihydroxy-16a-methylpregna-1,4-diene-3,20-dione 21-pivalate [34097-16-0].
»Clocortolone Pivalate contains not less than 97.0percent and not more than 103.0percent of C27H36ClFO5,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Color and clarity of solution—
A1in 100solution in chloroform is clear and practically colorless.
Identification—
A:
Infrared Absorption á197Mñ.
B:
Ultraviolet Absorption á197Uñ—
Solution:
15µg per mL.
Medium:
methanol.
Absorptivities at 238nm,calculated on the dried basis,do not differ by more than 3.0%.
Specific rotation á781Sñ:
between +125 and +135
and +135 .
.
Test solution:
40mg per mL,in chloroform.
Loss on drying á731ñ—
Dry it at 105 for 3hours:it loses not more than 1.0%of its weight.
for 3hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.2%,a 100-mg test specimen being used.
Chromatographic purity—
Test solution—
Accurately weigh about 100mg of Clocortolone Pivalate,and transfer to a 25-mLvolumetric flask.Dissolve in a mixture of chloroform and methanol (1:1),and dilute with the same solvent to volume.
Standard solution—
Using an accurately weighed quantity of USP Clocortolone Pivalate RS,prepare a solution in a mixture of chloroform and methanol (1:1)having a known concentration of about 4mg per mL.
Procedure—
Score a 20-×20-cm thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture into three equal sections to be used for the Test solution,the blank,and the Standard solution,respectively.Activate the plate at 105 for 30minutes before use.Apply 100µLeach of the Test solutionand the Standard solutionas streaks 2.5cm from the bottom of the appropriate section of the plate,and dry the streaks with a gentle current of air.Using a solvent system consisting of a mixture of cyclohexane and ethyl acetate (2:1),develop the chromatogram in a suitable chromatographic chamber lined with absorbent paper and previously equilibrated,until the solvent front has moved 15cm above the line of application.Air-dry the plate,and develop the chromatogram a second time using the same chromatographic system.Air-dry the plate,and locate the principal band occupied by the Standard solutionby viewing under UVlight.Mark this band as well as corresponding bands in the blank and Test solutionsections of the plate.Quantitatively remove the silica gel from each band,and transfer to separate glass-stoppered,50-mLcentrifuge tubes.Add 25.0mLof methanol to each tube,shake for not less than 20minutes,and centrifuge.Concomitantly determine the absorbances of the supernatants from the Test solutionand the Standard solutionagainst the blank at the wavelength of maximum absorbance at about 238nm,with a suitable spectrophotometer.Calculate the percentage of chromatographic impurities in the Test solutiontaken by the formula:
for 30minutes before use.Apply 100µLeach of the Test solutionand the Standard solutionas streaks 2.5cm from the bottom of the appropriate section of the plate,and dry the streaks with a gentle current of air.Using a solvent system consisting of a mixture of cyclohexane and ethyl acetate (2:1),develop the chromatogram in a suitable chromatographic chamber lined with absorbent paper and previously equilibrated,until the solvent front has moved 15cm above the line of application.Air-dry the plate,and develop the chromatogram a second time using the same chromatographic system.Air-dry the plate,and locate the principal band occupied by the Standard solutionby viewing under UVlight.Mark this band as well as corresponding bands in the blank and Test solutionsections of the plate.Quantitatively remove the silica gel from each band,and transfer to separate glass-stoppered,50-mLcentrifuge tubes.Add 25.0mLof methanol to each tube,shake for not less than 20minutes,and centrifuge.Concomitantly determine the absorbances of the supernatants from the Test solutionand the Standard solutionagainst the blank at the wavelength of maximum absorbance at about 238nm,with a suitable spectrophotometer.Calculate the percentage of chromatographic impurities in the Test solutiontaken by the formula:
100-[100(CS/CU)(AU/AS)],
in which CSis the concentration,in mg per mL,of USP Clocortolone Pivalate RSin the Standard solution,CUis the concentration,in mg per mL,of the Test solution,and AUand ASare the absorbances of the solutions from the Test solutionand the Standard solution,respectively:not more than 3.0%of total impurities is found.
Assay—
Standard preparation—
Dissolve an accurately weighed quantity of USP Clocortolone Pivalate RSin chloroform to obtain a solution having a known concentration of about 0.75mg per mL.Dilute an accurately measured volume of this solution with methanol,and mix to obtain a Standard preparationhaving a known concentration of about 30µg per mL.
Assay preparation—
Accurately weigh about 75mg of Clocortolone Pivalate,and transfer to a 100-mLvolumetric flask.Dissolve in chloroform,dilute with chloroform to volume,and mix.Transfer 4.0mLof this solution to a 100-mLvolumetric flask,dilute with methanol to volume,and mix.
Procedure—
Transfer 10.0-mLportions of the Standard preparationand the Assay preparationto separate glass-stoppered,50-mLconical flasks,and evaporate on a steam bath to dryness.To each flask,and to a third flask to provide the blank,add 15.0mLof a solution containing 250mg of isoniazid and 0.3mLof hydrochloric acid in 500mLof methanol.Swirl the contents of the flasks to dissolve the residues.Insert the stoppers securely in the flasks,and place in a water bath at 60 for 2.5hours.Cool to room temperature.Concomitantly determine the absorbances of the Assay preparationand the Standard preparationin 1-cm cells against the blank at the wavelength of maximum absorbance at about 405nm,with a suitable spectrophotometer.Calculate the quantity,in mg,of C27H36ClFO5in the portion of Clocortolone Pivalate taken by the formula:
for 2.5hours.Cool to room temperature.Concomitantly determine the absorbances of the Assay preparationand the Standard preparationin 1-cm cells against the blank at the wavelength of maximum absorbance at about 405nm,with a suitable spectrophotometer.Calculate the quantity,in mg,of C27H36ClFO5in the portion of Clocortolone Pivalate taken by the formula:
2.5C(AU/AS),
in which Cis the concentration,in µg per mL,of USP Clocortolone Pivalate RSin the Standard preparation,and AUand ASare the absorbances of the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 504
Phone Number:1-301-816-8139