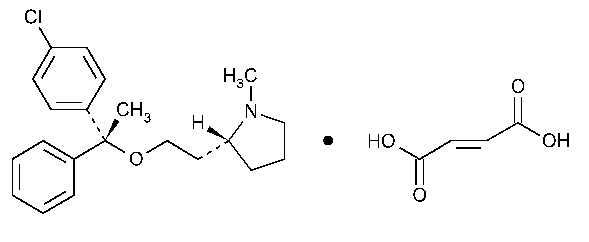
Clemastine Fumarate
Pyrrolidine,2-[2-[1-(4-chlorophenyl)-1-phenylethoxy]ethyl]-1-methyl-,[R-(R*,R*)]-,(E)-2-butenedioate (1:1).
(+)-(2R)-2-[2-[[[(R)-p-Chloro-a-methyl-a-phenylbenzyl]-oxy]ethyl]-1-methylpyrrolidine fumarate (1:1) [14976-57-9].
»Clemastine Fumarate contains not less than 98.0percent and not more than 102.0percent of C21H26ClNO·C4H4O4,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers,at a temperature not exceeding 25 .
.
Clarity and color of solution—
Dissolve 100mg of Clemastine Fumarate in 10.0mLof methanol,and mix to obtain the Test solution.Prepare a Comparison solutionby mixing 2.5mLof 0.00002Msodium chloride,2.5mLof water,5.0mLof 2.5Nnitric acid,and 1.0mLof 0.1Nsilver nitrate,and use this solution within 5minutes.Prepare a Color matching fluidby mixing 1volume of Matching Fluid C(see Color and Achromicity á631ñ)with 3volumes of water.Transfer the Test solution,the Comparison solution,and 10mLof Color matching fluidto separate test tubes having the same nominal diameter (about 12mm).View the Test solutionand the Comparison solutionhorizontally against a dull black background:the Test solutionis clear or not more opalescent than the Comparison solution.View the Test solutionand Color matching fluidhorizontally against a dull white background:the Test solutionis colorless or not more intensely colored than Color matching fluid.
Identification—
A:
Infrared Absorption á197Mñ.
B:
Prepare a Test preparationby dissolving 40mg of Clemastine Fumarate in 2.0mLof dilute alcohol (8in 10)with slight warming.Similarly prepare a Standard preparationby dissolving 50mg of fumaric acid in 10.0mLof dilute alcohol (8in 10).Separately apply 5-µLportions of the Test preparationand the Standard preparationto a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture,and dry the spots with the aid of a current of air.Develop the chromatogram in a solvent system consisting of a mixture of diisopropyl ether,formic acid,and water (70:25:5)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,dry at 100 for 30minutes,cool,and spray the plate with 0.1Mpotassium permanganate.Dry briefly with the aid of a current of warm air,and examine the chromatogram:the principal spot obtained from the Test preparationcorresponds in RFvalue,color,and intensity to that obtained from the Standard preparation.
for 30minutes,cool,and spray the plate with 0.1Mpotassium permanganate.Dry briefly with the aid of a current of warm air,and examine the chromatogram:the principal spot obtained from the Test preparationcorresponds in RFvalue,color,and intensity to that obtained from the Standard preparation.
Specific rotation á781Sñ:
between +15.0 and +18.0
and +18.0 (t=20
(t=20 ).
).
Test solution:
10mg per mL,in methanol.
pHá791ñ:
between 3.2and 4.2,in a suspension (1in 10).
Loss on drying á731ñ—
Dry it at 105 to constant weight:it loses not more than 0.5%of its weight.
to constant weight:it loses not more than 0.5%of its weight.
Heavy metals,Method IIá231ñ:
0.002%.
Chromatographic purity—
Spray reagent—
Dissolve 850mg of bismuth subnitrate in a mixture of 10mLof glacial acetic acid and 40mLof water,and mix (Solution A).Dissolve 8g of potassium iodide in 20mLof water (Solution B).Mix 5.0of Solution A,5.0mLof Solution B,and 20mLof glacial acetic acid in a 100-mLvolumetric flask,dilute with water to volume,and mix.
Standard preparation—
Dissolve a suitable quantity of USP Clemastine Fumarate RSin a mixture of chloroform and methanol (1:1)to obtain a solution having a known concentration of 20mg per mL.Dilute portions of this solution quantitatively with the mixture of chloroform and methanol (1:1)to prepare 5Comparison solutionshaving known concentrations of 0.10,0.08,0.06,0.04,and 0.02mg per mL,respectively (0.5%,0.4%,0.3%,0.2%,and 0.1%of the Standard preparation,respectively).
Test preparation—
Dissolve 100mg of Clemastine Fumarate in 5.0mLof a mixture of chloroform and methanol (1:1),and mix.
Procedure—
Separately apply 5-µLportions of the Standard preparation,each of the 5Comparison solutions,and the Test preparationto a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Allow the spots to dry,and develop the chromatogram in a solvent system consisting of a mixture of chloroform,methanol,and ammonium hydroxide (90:10:1)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,and dry the plate at room temperature with the aid of a current of air.Locate the spots on the plate by spraying first with Spray reagent,then with 3%hydrogen peroxide:the principal spot obtained from the Test preparationcorresponds in RFvalue,color,and intensity to that obtained from the Standard preparation;the sum of the intensities of any secondary spots,if present in the chromatogram from the Test preparation,corresponds to not more than 1.0%;and the intensities of any secondary spots do not exceed 0.5%of that of the principal spot in the chromatogram from the Standard preparationon the basis of comparison with spots obtained from the Comparison solutions.
Assay—
Transfer about 350mg of Clemastine Fumarate,accurately weighed,to a small conical flask,and dissolve in 60mLof glacial acetic acid.Titrate with 0.1Nperchloric acid VS,determining the endpoint potentiometrically.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 46.00mg of C21H26ClNO·C4H4O4.
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 490
Phone Number:1-301-816-8379