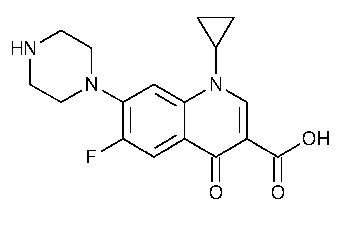
Ciprofloxacin
3-Quinolinecarboxylic acid,1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-.
1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid [85721-33-1].
»Ciprofloxacin contains not less than 98.0percent and not more than 102.0percent of C17H18FN3O3,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.Store at 25 ,excursion permitted between 15
,excursion permitted between 15 and 30
and 30 ,and avoid excessive heat.
,and avoid excessive heat.
Labeling—
Where it is intended for use in preparing injectable dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.Where it is intended for use in preparing Ciprofloxacin for Oral Suspension,it is so labeled.
USP Reference standards á11ñ—
USP Ciprofloxacin RS.USP Ciprofloxacin Ethylenediamine Analog RS.USP Fluoroquinolonic Acid RS.
Clarity of solution—
Dissolve 0.25g in 10mLof 0.1Nhydrochloric acid:a clear to slightly opalescent solution is obtained.
Identification—
A:
The IRabsorption spectrum of a potassium bromide dispersion of it exhibits maxima at the same wavelengths as that of a similar preparation of USP Ciprofloxacin RS.
B:
Dissolve a quantity of Ciprofloxacin in 6Nammonium hydroxide to obtain a test solution containing 10.0mg per mL.Dissolve a quantity of USP Ciprofloxacin RSin 6Nammonium hydroxide to obtain a Standard solution containing 10.0mg per mL.Proceed as directed for Identificationtest Bunder Ciprofloxacin Hydrochloride,beginning with “Separately apply.”The specified results are obtained.
Microbial limits á61ñ—
Where it is intended for use in preparing Ciprofloxacin for Oral Suspension,the total microbial count does not exceed 1000cfu per g,and the total combined molds and yeast count does not exceed 100cfu per g.It also meets the requirement for absence of Salmonellaand Escherichia coli.
Loss on drying á731ñ—
Dry it in vacuum at 120 for 6hours:it loses not more than 1.0%of its weight,except that where it is labeled as intended for use in preparing Ciprofloxacin for Oral Suspension,it loses between 10%and 20%of its weight.
for 6hours:it loses not more than 1.0%of its weight,except that where it is labeled as intended for use in preparing Ciprofloxacin for Oral Suspension,it loses between 10%and 20%of its weight.
Residue on ignition á281ñ:
not more than 0.1%,except that where it is intended for use in preparing Ciprofloxacin for Oral Suspension,it is not more than 0.2%.
Chloride—
Add 30.0mLof water to 0.5g of Ciprofloxacin,shake for 5minutes,and filter through chloride-free filter paper.Transfer 15.0mLof the filtrate to a 50-mLcolor-comparison tube (test solution).To a second matched 50-mLcolor-comparison tube transfer 10.0mLof a standard solution of sodium chloride having a concentration of 8.2µg per mL,corresponding to 5µg of chloride per mL,add 5.0mLof water,and mix.To each tube add 1mLof 2Nnitric acid,mix,add 1mLof silver nitrate TS,and mix.The turbidity exhibited by the test solution does not exceed that of the standard solution (0.02%).
Sulfate—
Dissolve 0.5g in 5.0mLof 2Nacetic acid and 15.0mLof water (test solution).To each of two 50-mLmatched color-comparison tubes transfer 1.50mLof a standard solution of potassium sulfate in 30%alcohol having a concentration of 18.1µg per mL,equivalent to 10µg of sulfate per mL.To each tube add,successively and with continuous shaking,1.0mLof barium chloride solution (1in 4),and allow to stand for 1minute.To one of the tubes transfer 15.0mLof the standard solution and 0.5mLof 30%acetic acid,and mix.To the second tube add 15.0mLof the test solution and 0.5mLof 30%acetic acid,and mix:the turbidity exhibited in the tube containing the test solution does not exceed that of the tube containing the standard solution (0.04%).
Heavy metals,Method IIá231ñ:
0.002%.
Limit of fluoroquinolonic acid—
Dissolve a quantity of Ciprofloxacin in 0.1Nacetic acid to obtain a test solution containing 10.0mg per mL.Proceed as directed in the test for Limit of fluoroquinolonic acidunder Ciprofloxacin Hydrochloride,beginning with “Transfer 5.0mg of USP Fluoroquinolonic Acid RS.”The specified result is obtained.
Chromatographic purity—
Mobile phase,Standard preparation,Resolution solution,Assay preparation,andChromatographic system—
Prepare as directed in the Assay.
Procedure—
Proceed as directed for Procedurein the Assay.Calculate the percentage of each impurity peak in the chromatogram obtained from the Assay preparationtaken by the formula:
100ri/rt,
in which riis the response of each impurity peak;and rtis the sum of the responses of all the peaks:not more than 0.2%of ciprofloxacin ethylenediamine analog or of any other individual impurity peak is found;and the sum of all the impurity peaks is not more than 0.5%.
Other requirements—
Where the label states that it is sterile,it meets the requirements for Sterility Tests á71ñand Pyrogenunder Ciprofloxacin Injection.Where the label states that Ciprofloxacin must be subjected to further processing during the preparation of injectable dosage forms,it meets the requirements for Pyrogen under Ciprofloxacin Injection.
Assay—
Mobile phase—
Prepare a filtered and degassed mixture of 0.025Mphosphoric acid,previously adjusted with triethylamine to a pHof 3.0±0.1,and acetonitrile (87:13).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Transfer about 12.5mg of USP Ciprofloxacin RS,accurately weighed,to a 25-mLvolumetric flask.Add 0.1mLof 7%phosphoric acid,dilute with Mobile phaseto volume,and mix.
Resolution solution—
Dissolve a quantity of USP Ciprofloxacin Ethylenediamine Analog RSin the Standard preparationto obtain a solution containing about 0.5mg per mL.
Assay preparation—
Transfer about 25mg of Ciprofloxacin,accurately weighed,to a 50-mLvolumetric flask.Add 0.2mLof 7%phosphoric acid,dilute with Mobile phaseto volume,and mix.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 278-nm detector and a 4-mm ×25-cm column that contains packing L1and is maintained at a temperature of 30±1 .The flow rate is about 1.5mLper minute.Chromatograph the Resolution solution,and record the responses as directed for Procedure:the retention time for ciprofloxacin is between 6.4and 10.8minutes;the relative retention times are about 0.7for ciprofloxacin ethylenediamine analog and 1.0for ciprofloxacin;and the resolution,R,between the ciprofloxacin ethylenediamine analog peak and the ciprofloxacin peak is not less than 6.Chromatograph the Standard preparation,and record the responses as directed for Procedure:the column efficiency,determined from the ciprofloxacin peak,is not less than 2500theoretical plates;the tailing factor for the ciprofloxacin peak is not more than 4.0;and the relative standard deviation for replicate injections is not more than 1.5%.
.The flow rate is about 1.5mLper minute.Chromatograph the Resolution solution,and record the responses as directed for Procedure:the retention time for ciprofloxacin is between 6.4and 10.8minutes;the relative retention times are about 0.7for ciprofloxacin ethylenediamine analog and 1.0for ciprofloxacin;and the resolution,R,between the ciprofloxacin ethylenediamine analog peak and the ciprofloxacin peak is not less than 6.Chromatograph the Standard preparation,and record the responses as directed for Procedure:the column efficiency,determined from the ciprofloxacin peak,is not less than 2500theoretical plates;the tailing factor for the ciprofloxacin peak is not more than 4.0;and the relative standard deviation for replicate injections is not more than 1.5%.
Procedure—
Separately inject equal volumes (about 10µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the areas for the major peaks.Calculate the quantity,in mg,of C17H18FN3O3in the portion of Ciprofloxacin taken by the formula:
50C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Ciprofloxacin RSin the Standard preparation;and rUand rSare the ciprofloxacin peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 476
Pharmacopeial Forum:Volume No.29(6)Page 1860
Phone Number:1-301-816-8394