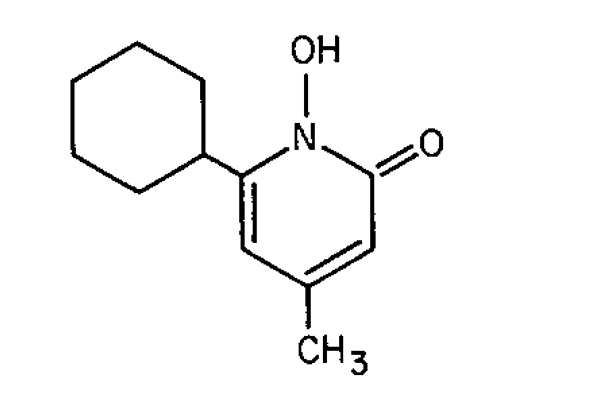
Ciclopirox
2(1H)-Pyridinone,6-cyclohexyl-1-hydroxy-4-methyl-.
6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone [29342-05-0].
»Ciclopirox contains not less than 98.0percent and not more than 101.0percent of C12H17NO2,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers,protected from light.Store at a temperature between 15 and 30
and 30 .
.
USP Reference standards á11ñ—
USP Ciclopirox RS.USP Ciclopirox Related Compound A RS.USP Ciclopirox Related Compound B RS.
Identification,
Infrared Absorption á197Kñ.
Loss on drying á731ñ—
Dry it in vacuum to constant weight:it loses not more than 1.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
not more than 0.001%.
Related compounds—
[NOTE—Carry out the operations avoiding exposure to actinic light.All materials in direct connection with ciclopirox,like column materials,reagents,solvents,and others should contain only very low amounts of extractable metal cations.]
Mobile phase—
Prepare a filtered and degassed mixture of an edetate disodium solution (0.96in 1000),acetonitrile,and glacial acetic acid (770:230:0.1).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Rinsing solution—
Prepare a mixture of water,acetonitrile,glacial acetic acid,and acetylacetone (500:500:1:1).
Standard stock solution—
Dissolve 15mg of USP Ciclopirox Related Compound A RSand 15mg of USP Ciclopirox Related Compound B RS,accurately weighed,in 1mLof acetonitrile and 7mLof Mobile phase.Dilute the solution thus obtained with Mobile phase to 10.0mLin order to obtain a solution having a known concentration of 1.5mg each per mL.
Standard solution A—
Dilute 1.0mLof Standard stock solution to 200.0mLwith a mixture of Mobile phase and acetonitrile (9:1).
Standard solution B—
Dilute 2.0mLofStandard solution Ato 10.0mLwith a mixture of Mobile phaseand acetonitrile (9:1).
Test solution—
Dissolve 30mg of ciclopirox,accurately weighed,in a mixture of 2mLof acetonitrile and 15mLof Mobile phase.If necessary,use an ultrasonic bath.Dilute with Mobile phase to 20.0mL.
Resolution solution—
Mix 5mLof Standard stock solutionwith 5mLof the Test solution.
Chromatographic system (seeChromatography á621ñ)—
The liquid chromatograph is equipped with a detector capable of recording at both 220nm and 298nm and a 4.0-mm x 8-cm column that contains packing L10.[NOTE—Ciclopirox related compound Ahas an intense absorbance at 220nm,and 6-cyclohexyl-4-methyl-2(1H)-pyridone,ciclopirox related compound B,and ciclopirox have intense absorbances at 298nm.]The flow rate is about 0.7mLper minute.Chromatograph the Resolution solution,and record the peak responses as directed for Procedureat 298nm:the resolution,R,between the ciclopirox related compound Bpeak and ciclopirox peak is not less than 2.0.Chromatograph the Standard solution B,and record the peak responses as directed for Procedureat 298nm:the chromatogram obtained shows at 298nm a peak corresponding to ciclopirox related compound Bwith a signal-to-noise ratio of not less than 3.Chromatograph the Test solution,and record the peak responses as directed for Procedureat 298nm:the tailing factor for the ciclopirox peak is less than 2.0.
Procedure—
Separately inject equal volumes (about 10µL)of Standard solution A,Standard solution B,and the Test solutioninto the chromatograph,and record the chromatograms.[NOTE—In order to ensure desorption of disruptive metal ions,every new column must be rinsed with the Rinsing solution over a period of not less than 15hours and then with the Mobile phasefor not less than 5hours with a flow rate of 0.2mLper minute.The chromatographic run time is not less than 2.5times the retention time of the ciclopirox peak.]The relative retention times are about 0.5for ciclopirox related compound A,0.9for 6-cyclohexyl-4-methyl-2(1H)-pyridone,1.0for ciclopirox,and 1.3for ciclopirox related compound B.The peak response at 220nm of the ciclopirox related compound Apeak in the chromatogram obtained from the Test solutionis not more than the peak response at 220nm of the corresponding peak in the chromatogram obtained from Standard solution A(0.5%).The sum of responses at 298nm of the peaks in the chromatogram obtained from the Test solutionis not more than the peak response at 298nm of the ciclopirox related compound Bpeak in the chromatogram obtained from Standard solution A(0.5%).At 298nm disregard any peak due to the solvent and any peak with a response less than the response of the ciclopirox related compound Bpeak in the chromatogram obtained from Standard solution Bat 298nm (0.1%).
Assay—
Dissolve 150mg of Ciclopirox,accurately weighed,in 20mLof methanol.Add 20mLof water,mix,and titrate with 0.1Nsodium hydroxide VS,determining the endpoint potentiometrically.Carry out a blank test.Determine the factor of the 0.1Nsodium hydroxide VSusing 100mg of benzoic acid,accurately weighed,and titrate under the conditions prescribed above.Each mLof 0.1Nsodium hydroxide is equivalent to 20.73mg of C12H17NO2.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 469
Pharmacopeial Forum:Volume No.29(2)Page 393
Phone Number:1-301-816-8394