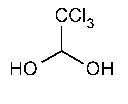
Chloral Hydrate
»Chloral Hydrate contains not less than 99.5percent and not more than 102.5percent of C2H3Cl3O2.
Packaging and storage—
Preserve in tight containers.
Identification—
Transfer to a 125-mLconical flask a portion of a solution in water equivalent to about 1mg of chloral hydrate,and add water to bring the volume to about 10mL.Add 10mLof 1-ethylquinaldinium iodide solution (15in 1000),which has been filtered through a 0.45-µm filter.Add 60mLof isopropyl alcohol,5mLof an aqueous 0.1Mmonoethanolamine solution,and 15mLof water.Mix,and heat in a water bath at 60 for 15minutes:a blue color develops.
for 15minutes:a blue color develops.
Acidity—
A1in 20solution in alcohol does not at once redden moistened blue litmus paper.
Residue on ignition á281ñ:
not more than 0.1%.
Chloride á221ñ—
To a 1in 10solution in alcohol add a few drops of silver nitrate TS:any opalescence produced does not exceed that of a control containing 0.10mLof 0.020Nhydrochloric acid (0.007%).
Readily carbonizable substances á271ñ—
Shake 500mg,at intervals of 5minutes during 1hour,with 5mLof sulfuric acid TSin a glass-stoppered cylinder that previously has been rinsed with sulfuric acid TS,and transfer the mixture to a comparison vessel:the mixture has no more color than Matching Fluid P.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Dissolve about 4g of Chloral Hydrate,accurately weighed,in 10mLof water,add 30.0mLof 1Nsodium hydroxide VS,and allow the mixture to stand for 2minutes.Add a few drops of phenolphthalein TS,and titrate the residual alkali at once with 1Nsulfuric acid VS.Each mLof 1Nsodium hydroxide corresponds to 165.4mg of C2H3Cl3O2.
Auxiliary Information—
Staff Liaison:Ravi Ravichandran,Ph.D.,Senior Scientist
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 425
Phone Number:1-301-816-8330