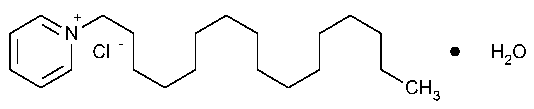
Cetylpyridinium Chloride
Pyridinium,1-hexadecyl-,chloride,monohydrate.
1-Hexadecylpyridinium chloride monohydrate [6004-24-6].
Anhydrous 339.99 [123-03-5].
»Cetylpyridinium Chloride contains not less than 99.0percent and not more than 102.0percent of C21H38ClN,calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
Infrared Absorption á197Kñ.
Solution:
40µg per mL.
Medium:
water.
C:
Dissolve 100mg in 50mLof water:a 10-mLportion of the solution responds to the tests for Chloride á191ñ,except that a turbidity is produced,rather than a curdy white precipitate,when the silver nitrate TSis added.
Acidity—
Dissolve 500mg,accurately weighed,in 50mLof water,add phenolphthalein TS,and titrate with 0.020Nsodium hydroxide:not more than 2.5mLis required for neutralization.
Water,Method Iá921ñ:
between 4.5%and 5.5%.
Residue on ignition á281ñ:
not more than 0.2%,calculated on the anhydrous basis.
Heavy metals,Method IIá231ñ:
0.002%.
Pyridine—
Dissolve 1g in 10mLof sodium hydroxide solution (1in 10)without heating:the odor of pyridine is not immediately perceptible.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Assay—
Transfer about 200mg of Cetylpyridinium Chloride,accurately weighed,to a glass-stoppered,250-mLgraduated cylinder containing 75mLof water.Add 10mLof chloroform,0.4mLof bromophenol blue solution (1in 2000),and 5mLof a freshly prepared solution of sodium bicarbonate (4.2in 1000),and titrate with 0.02Msodium tetraphenylboron VSuntil the blue color disappears from the chloroform layer.Add the last portions of the sodium tetraphenylboron solution dropwise,agitating vigorously after each addition.Each mLof 0.02Msodium tetraphenylboron is equivalent to 6.800mg of C21H38ClN.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 423
Phone Number:1-301-816-8394