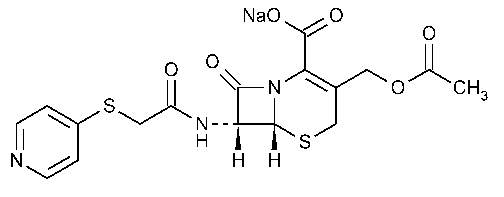
Cephapirin Sodium
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid,3-[(acetyl-oxy)methyl]-8-oxo-7-[[(4-pyridinylthio)acetyl]amino]-,monosodium salt,(6R-trans)-.
Monosodium (6R,7R)-3-(hydroxymethyl)-8-oxo-7-[2-(4-pyridylthio)acetamido]-5-thia-1-azabicyclo-[4.2.0]oct-2-ene-2-carboxylate acetate (ester)
[24356-60-3].
»Cephapirin Sodium has a potency equivalent to not less than 855µg and not more than 1000µg of cephapirin (C17H17N3O6S2)per mg.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification—
A:
Infrared Absorption á197Kñ.
B:
It responds to the tests for Sodium á191ñ.
Crystallinity á695ñ:
meets the requirements.
pHá791ñ:
between 6.5and 8.5,in a solution containing 10mg of cephapirin per mL.
Water,Method Iá921ñ:
not more than 2.0%.
Other requirements—
Where the label states that Cephapirin Sodium is sterile,it meets the requirements for Sterilityand Bacterial endotoxinsunder Cephapirin for Injection.Where the label states that Cephapirin Sodium must be subjected to further processing during the preparation of injectable dosage forms,it meets the requirements for Bacterial endotoxinsunder Cephapirin for Injection.
Change to read:
Assay—
Assay preparation—
In duplicate,weigh about 50mg of Cephapirin Sodium,and transfer into a 25-mLvolumetric flask.Add about 2.5mLof Extraction solutionand 15.0mLof Dilution buffer,and mix to dissolve.Add 7.0mLof acetonitrile,and mix.Allow the flask to return to room temperature,and dilute with water to volume.
Procedure—
Separately inject equal volumes (about 2µL)of the duplicate Standard preparationand the duplicate Assay preparationinto the chromatograph,record the chromatograms,and measure the areas for the major peaks.Calculate the quantity,in µg,of cephapirin (C17H17N3O6S2)in each mg of Cephapirin Sodium taken by the formula:
 USP28
USP28
P(WS/WU)(VU/VS)(rU/rS),
in which Pis the assigned potency,in µg of cephapirin per mg,of USP Cephapirin Sodium RS;WSand WUare the quantities of USP Cephapirin Sodium RSand Cephapirin Sodium,in mg,used to prepare the Standard preparation and the Assay preparation,respectively;VUand VSare the final volumes,in mL,of the Assay preparation and the Standard preparation,respectively;and rUand rSare the average peak areas of the cephapirin peaks obtained from the Assay preparation and the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Ian DeVeau,Ph.D.,Senior Scientist
Expert Committee:(VET)Veterinary Drugs
USP28–NF23Page 419
Pharmacopeial Forum:Volume No.30(2)Page 471
Phone Number:1-301-816-8178