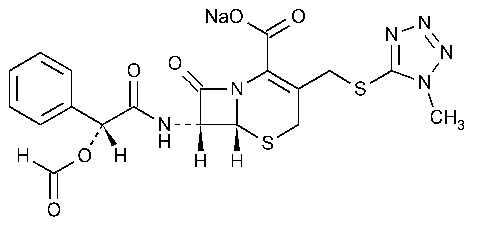
Cefamandole Nafate
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid,7-[[(formyloxy)phenylacetyl]amino]-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-,monosodium salt,[6R-[6a,7b(R*)]]-.
Sodium (6R,7R)-7-(R)-mandelamido-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate formate (ester) [42540-40-9].
»Cefamandole Nafate has a potency equivalent to not less than 810µg and not more than 1000µg of cefamandole (C18H18N6O5S2)per mg,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification—
Prepare a mixture of ethyl acetate,acetone,glacial acetic acid,and water (5:2:1:1)(Developing solvent).Prepare a solution of the specimen inDeveloping solvent containing 10mg per mL.Prepare a Standard solution of USP Cefamandole Nafate RSin Developing solventcontaining 10mg per mL.Use these solutions promptly after preparation.Apply separately 10µLof each solution to a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Place the plate in a suitable chromatographic chamber,previously equilibrated with Developing solventfor not less than 30minutes,and develop the chromatogram until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,and allow to air-dry.Locate the spots on the plate by examination under short-wavelength UVlight:the RFvalue of the principal spot obtained from the test solution corresponds to that obtained from the Standard solution.
pHá791ñ:
between 3.5and 7.0,in a solution containing 100mg per mL.
Water,Method Iá921ñ:
not more than 2.0%.
Other requirements—
Where the label states that Cefamandole Nafate is sterile,it meets the requirements for Sterility Tests á71ñand for Bacterial endotoxinsunder Cefamandole Nafate for Injection.Where the label states that Cefamandole Nafate must be subjected to further processing during the preparation of injectable dosage forms,it meets the requirements for Bacterial endotoxinsunder Cefamandole Nafate for Injection.
Assay—
pH2.3buffer—
Dissolve 3.6g of anhydrous dibasic sodium phosphate,39.4g of citric acid monohydrate,and 70.8g of potassium chloride in water to make 1000mL.
Standard preparation—
Transfer about 12mg of USP Cefamandole Nafate RS,accurately weighed,to a 50-mLvolumetric flask containing 4mLof water.Immediately before use,add 30.0mLof pH2.3buffer,dilute with water to volume,and mix.
Assay preparation—
Using Cefamandole Nafate,prepare as directed under Standard preparation.
Procedure—
Transfer a portion of the Assay preparationto a suitable polarographic cell.Deaerate by bubbling scrubbed nitrogen through the solution for 5minutes,and redirect the nitrogen flow to the surface outlet.Insert the dropping mercury electrode of a suitable polarograph (see Polarography á801ñ)capable of measuring a current of 0.5microampere or appropriate current to maintain on-scale response,using an average capillary,and a drop rate of 1per second.Record the polarogram in the differential pulse mode from -0.3volt to -1.05volts,using a saturated calomel reference electrode and platinum wire counter electrode.Determine the peak height obtained,in microamperes,where the peak height is defined as the perpendicular distance from the extrapolated baseline to the highest point of the peak as compared to the full-scale current range.Similarly,determine the peak current of the Standard preparation.Calculate the quantity,in µg,of C18H18N6O5S2in each mg of the Cefamandole Nafate taken by the formula:
P(WS/WU)(iU/iS),
in which Pis the potency,in µg of cefamandole per mg,of USP Cefamandole Nafate RS,WSand WUare the quantities,in mg,of USP Cefamandole Nafate RSand Cefamandole Nafate taken to prepare the Standard preparationand the Assay preparation,respectively,and iUand iSare the peak currents,in microamperes,from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 373
Phone Number:1-301-816-8335