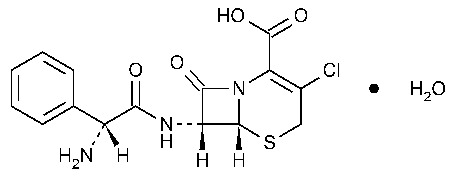
Cefaclor
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid,7-[(aminophenylacetyl)amino]-3-chloro-8-oxo-,monohydrate,[6R-[6a,-7b(R*)]]-.
(6R,7R)-7-[(R)-2-Amino-2-phenylacetamido]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid monohydrate.
3-Chloro-7-D-(2-phenylglycinamido)-3-cephem-4-carboxylic acid monohydrate [70356-03-5].
Anhydrous 367.81 [53994-73-3].
»Cefaclor has a potency of not less than 950µg and not more than 1020µg of C15H14ClN3O4Sper mg,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
Infrared Absorption á197Kñ.
B:
The retention time of the major peak for cefaclor in the chromatogram of the Assay corresponds to that in the chromatogram of the Standard preparation,as obtained in the Assay.
Crystallinity á695ñ:
meets the requirements.
pHá791ñ:
between 3.0and 4.5,in an aqueous suspension containing 25mg per mL.
Water,Method Iá921ñ:
between 3.0%and 6.5%.
Related compounds—
Solvent—
Dissolve 2.4g of monobasic sodium phosphate in 1000mLof water,and adjust with phosphoric acid to a pHof 2.5.
Blank solution—
Use the Solvent.
Solution A—
Dissolve 6.9g of monobasic sodium phosphate in 1000mLof water,and adjust with phosphoric acid to a pHof 4.0.
Solution B—
Prepare a mixture of Solution Aand acetonitrile (550:450),degassing for not more than 2minutes.
Mobile phase—
Use variable mixtures of Solution Aand Solution Bas directed for Chromatographic system.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).[NOTE—Reducing the acetonitrile content increases the retention time of cefaclor and increases the resolution between cefaclor,delta-3isomer and cefaclor.]
Standard solution—
Dissolve an accurately weighed quantity of USP Cefaclor RSin Solventto obtain a solution having a known concentration of about 0.05mg per mL.Sonicate briefly,if necessary,to dissolve,and avoid heating.[NOTE—Use this solution on the day it is prepared.]
System suitability solution—
Dissolve a quantity of USP Cefaclor,Delta-3Isomer RSin the Standard solutionto obtain a solution having a concentration of about 0.05mg per mL.
Test solutions—
Transfer about 50mg of Cefaclor,accurately weighed,to each of two 10-mLvolumetric flasks,dilute each with Solventto volume,and mix.Sonicate briefly,if necessary,to dissolve,and avoid heating.[NOTE—Use these Test solutionswithin 2hours when stored at room temperature or within 20hours when stored under refrigeration.]
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm ×25-cm column that contains packing L1.The flow rate is about 1mLper minute.The chromatograph is programmed as follows.
Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the retention time for the cefaclor peak is between 23and 29minutes;the resolution,R,between cefaclor,delta-3isomer and cefaclor is not less than 2.0;and the tailing factor for the cefaclor peak is not more than 1.2.Chromatograph the Blank solutionas directed for Procedure.Examine the chromatogram for any extraneous peaks,and disregard any corresponding peaks observed in the chromatogram of the Test solutions.[NOTE—Ensure that any extraneous peaks observed do not represent carryover from previous injections.]
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0 | 95 | 5 | equilibration |
| 0–30 | 95®75 | 5®25 | linear gradient |
| 30–45 | 75®0 | 25®100 | linear gradient |
| 45–55 | 0 | 100 | isocratic |
| 55–60 | 0®95 | 100®5 | reset composition |
| 60–70 | 95 | 5 | re-equilibration |
Procedure—
Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutionsinto the chromatograph,record the chromatograms,and measure all of the peak areas.Calculate the percentage of each cefaclor related compound in the portion of Cefaclor taken by the formula:
(CP/W)(ri/rS),
in whichCis the concentration,in mg per mL,of USP Cefaclor RSin theStandard solution;Pis the designated potency,in µg per mg,of USP Cefaclor RS;Wis the weight,in mg,of the portion of Cefaclor taken to prepare the respective Test solution;riis the peak response of an individual related compound in the chromatogram obtained from the Test solution;and rSis the peak response for the cefaclor peak in the chromatogram of the Standard solution.Determine the mean values for each cefaclor related compound:not more than 0.5%of any individual cefaclor related compound is found,and not more than 2.0%of total cefaclor related compounds is found.In an acceptable determination,the difference between duplicate determinations of total cefaclor related compounds is not more than 0.2%absolute,or the variation from the mean of the two values is not more than 10%,whichever is greater.
Assay—
Mobile phase—
Dissolve 1g of sodium 1-pentanesulfonate in a mixture of 780mLof water and 10mLof triethylamine.Adjust with phosphoric acid to a pHof 2.5±0.1,add 220mLof methanol,and mix.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Transfer about 15mg of USP Cefaclor RS,accurately weighed,to a 50-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.Sonicate briefly,if necessary,to achieve dissolution,and avoid heating the solution.[NOTE—Use this Standard preparation within 8hours if stored at room temperature,or within 20hours if stored under refrigeration.]
Assay preparation—
Transfer about 15mg of Cefaclor,accurately weighed,to a 50-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.Sonicate briefly,if necessary,to achieve dissolution,and avoid heating the solution.[NOTE—Use this Assay preparation within 8hours if stored at room temperature,or within 20hours if stored under refrigeration.]
Resolution solution—
Prepare a solution in Mobile phasecontaining about 0.3mg of cefaclor and 0.3mg of USP Cefaclor,Delta-3Isomer RSper mL.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 265-nm detector and a 4.6-mm ×25-cm column containing 5-µm packing L1.The flow rate is about 1.5mLper minute.Chromatograph the Resolution solution,and record the responses as directed for Procedure:the relative retention times for cefaclor and cefaclor,delta-3isomer are about 0.8and 1.0,the resolution,R,between the cefaclor peak and the cefaclor,delta-3isomer peak is not less than 2.5,the tailing factor is not more than 1.5,and the relative standard deviation for replicate injections is not more than 2%.
Procedure—
[NOTE—Use peak areas where peak responses are indicated.]Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay into the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the potency,in µg per mg,of cefaclor (C15H14ClN3O4S)in each mg of the Cefaclor taken by the formula:
(WS/WU)(P)(rU/rS),
in which WSand WUare the weights,in mg,of USP Cefaclor RSand of Cefaclor taken to prepare the Standard preparationand the Assay preparation,respectively;Pis the designated potency,in µg of cefaclor (C15H14ClN3O4S)per mg,of USP Cefaclor RS;and rUand rSare the peak responses of the cefaclor peaks obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 369
Pharmacopeial Forum:Volume No.28(4)Page 1084
Phone Number:1-301-816-8335