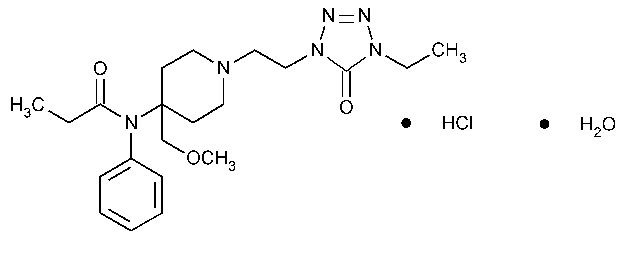
Alfentanil Hydrochloride
Propanamide,N-[1-[2-(4-ethyl-4,5-dihydro-5-oxo-1H-tetrazol-1-yl)ethyl]-4-(methoxymethyl)-4-piperidinyl]-N-phenyl,monohydrochloride,monohydrate.
N-[1-[2-(4-Ethyl-5-oxo-2-tetrazolin-1-yl)-ethyl]-4-(methoxymethyl)-4-piperidyl]propionanilide monohydrochloride monohydrate [70879-28-6;69049-06-5].
»Alfentanil Hydrochloride contains not less than 98.0percent and not more than 102.0percent of C21H32N6O3·HCl,calculated on the anhydrous basis.
Caution—Handle Alfentanil Hydrochloride with great care since it is a potent opioid analgesic.Great care should be taken to prevent inhaling particles of Alfentanil Hydrochloride and exposing the skin to it.
Packaging and storage—
Preserve in tight containers,and store at controlled room temperature.
Identification,
Infrared Absorption á197Kñ.
Water,Method Iá921ñ:
not more than 4.0%.
Residue on ignition á281ñ:
not more than 0.1%.
Chromatographic purity—
Mobile phase—
Prepare a filtered and degassed mixture of 0.01Mtetrabutylammonium hydrogen sulfate and acetonitrile (86:14).Make adjustments if necessary (seeSystem SuitabilityunderChromatography á621ñ).
Standard solution—
Dissolve an accurately weighed quantity of USP Alfentanil Hydrochloride RSinMobile phase,and dilute quantitatively,and stepwise if necessary,withMobile phase to obtain a solution having a known concentration of about 0.54mg per mL.
Test solution—
Transfer about 54mg of Alfentanil Hydrochloride,accurately weighed,to a 100-mLvolumetric flask,dilute withMobile phase to volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 235-nm detector and a 4.6-mm ×25-cm column that contains spherical 5-µm packing L1.The flow rate is about 2mLper minute.Chromatograph theStandard solution,and record the peak responses as directed underProcedure:the column efficiency is not less than 5400theoretical plates,the tailing factor is not more than 1.3,and the relative standard deviation for replicate injections is not more than 1.0%.
Procedure—
Inject a volume (about 25µL)of theTest solution into the chromatograph,record the chromatogram,and measure the responses for all of the peaks.Calculate the percentage of each impurity in the portion of Alfentanil Hydrochloride taken by the formula:
100(ri/rs),
in whichriis the response of each impurity peak,andrsis the sum of all of the peaks:not more than 0.5%of any single impurity is found,and the sum of all impurities is not more than 1.0%.
Assay—
Dissolve about 350mg of Alfentanil Hydrochloride,accurately weighed,in 30mLof glacial acetic acid.Add 3mLof mercuric acetate TSand 3drops of p-naphtholbenzein TS,and titrate with 0.1Nperchloric acid VSto a green endpoint.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 45.298mg of C21H32N6O3·HCl.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA2)Pharmaceutical Analysis 2
USP28–NF23Page 65
Pharmacopeial Forum:Volume No.29(6)Page 1834
Phone Number:1-301-816-8139