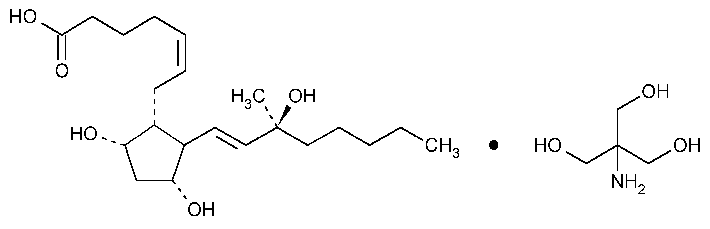
Carboprost Tromethamine
C21H36O5·C4H11NO3
489.64
Prosta-5,13-dien-1-oic acid,9,11,15-trihydroxy-15-methyl-,(5Z,-9a,11a,13E,15S)-,compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1).
(Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(E)-(3S)-3-hydroxy-3-methyl-1-octenyl]cyclopentyl]-5-heptenoic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1).
(15S)-15-Methylprostaglandin F2atromethamine [58551-69-2].
Prosta-5,13-dien-1-oic acid,9,11,15-trihydroxy-15-methyl-,(5Z,-9a,11a,13E,15S)-,compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1).
(Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(E)-(3S)-3-hydroxy-3-methyl-1-octenyl]cyclopentyl]-5-heptenoic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1).
(15S)-15-Methylprostaglandin F2atromethamine [58551-69-2].
»Carboprost Tromethamine contains not less than 95.0percent and not more than 105.0percent of C25H47NO8,calculated on the dried basis.
Caution—Great care should be taken to prevent inhaling particles of Carboprost Tromethamine and exposing the skin to it.
Packaging and storage—
Preserve in well-closed containers,and store in a freezer.
USP Reference standards á11ñ—
USP Carboprost Tromethamine RS.
Loss on drying á731ñ—
Dry it in vacuum at a pressure not exceeding 5mm of mercury at 50 for 16hours:it loses not more than 1.0%of its weight.
for 16hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.5%.
Limit of 15R-epimer and 5-trans isomer—
Mobile phase,Internal standard preparation,Citrate buffer,and Chromatographic system—
Proceed as directed in theAssay.To evaluate the system suitability requirements,use theStandard preparation prepared as directed in theAssay.
Test solution—
Use theAssay preparation.
Procedure—
Inject a volume (about 25µL)of theTest solution into the chromatograph,record the chromatogram,and measure the peak areas.Calculate the percentage of the 15R-epimer (as the tromethamine salt)in the portion of Carboprost Tromethamine taken by the formula:
100rA/(rA+rB+rC),
in which rAis the area of any peak at a relative retention time of 0.7;rBis the area of any peak at a relative retention time of 1.0;and rCis the area of any peak at a relative retention time of about 1.2.Not more than 2.0%is found.Calculate the percentage of the 5-transisomer (as the tromethamine salt)in the portion of Carboprost Tromethamine taken by the formula:
100rC/(rA+rB+rC),
in which the terms are as defined above.Not more than 3.0%is found.
Assay—
Mobile phase—
Prepare a filtered and degassed mixture of methylene chloride,1,3-butanediol,and water (992:7:0.5).Make adjustments if necessary (seeSystem Suitability underChromatography á621ñ).
Internal standard preparation—
Using theMobile phase,prepare a solution containing about 7mg of guaifenesin per mL.
Citrate buffer—
Dissolve 10.5g of citric acid in about 75mLof water.Add 5Nsodium hydroxide slowly to adjust to a pHof 4.0,and dilute with water to 100mL.
Standard preparation—
Transfer about 5mg of USP Carboprost Tromethamine RS,accurately weighed,to a stoppered,50-mLcentrifuge tube.Add 20.0mLof methylene chloride and 2mLofCitrate buffer.Shake the stoppered tube for about 10minutes,and centrifuge.Remove and discard the top (aqueous)layer,and transfer a 4.0-mLaliquot of the lower (methylene chloride)layer to a suitable vial.Evaporate with the aid of a stream of nitrogen to dryness.Add 100µLof a freshly prepared solution of a-bromo-2¢-acetonaphthone in acetonitrile (1in 50).Swirl to wash down the sides of the vial.Add 50µLof a freshly prepared solution of diisopropylethylamine in acetonitrile (1in 100),swirl again,and place the vial in a suitable heating device maintained at a temperature of 30 to 35
to 35 for not less than 15minutes.Evaporate the acetonitrile from the vial with the aid of a stream of nitrogen,add 2.0mLofInternal standard preparation,mix,and pass the resulting solution through a fine-porosity filter.Protect the filtered solution from light prior to injection to prevent degradation of the naphthacyl ester of carboprost.
for not less than 15minutes.Evaporate the acetonitrile from the vial with the aid of a stream of nitrogen,add 2.0mLofInternal standard preparation,mix,and pass the resulting solution through a fine-porosity filter.Protect the filtered solution from light prior to injection to prevent degradation of the naphthacyl ester of carboprost.
Assay preparation—
Proceed as directed forStandard preparation,except to use Carboprost Tromethamine in place of USP Carboprost Tromethamine RS.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm ×30-cm stainless steel column that contains 10-µm packing L3.The flow rate is about 1.8mLper minute.Chromatograph theStandard preparation,and record the responses as directed forProcedure:the relative retention times for guaifenesin and the 2-naphthacyl ester of carboprost are about 0.6and 1.0,respectively;the resolution,R,between guaifenesin and the 2-naphthacyl ester of carboprost is not less than 4.0;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10µL)of theStandard preparation and theAssay preparation into the chromatograph,record the chromatograms,and measure the peak responses.Calculate the quantity,in mg,of C25H47NO8in the portion of Carboprost Tromethamine taken by the formula:
W(RU/RS),
in whichWis the weight,in mg,of USP Carboprost Tromethamine RStaken to prepare theStandard preparation;and RUand RSare the peak response ratios of the 2-naphthacyl ester of carboprost to the internal standard obtained from theAssay preparation and theStandard preparation,respectively.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 357
Pharmacopeial Forum:Volume No.30(1)Page 82
Phone Number:1-301-816-8139